| | | | Notes to the 2016 Grants of Plan-Based Awards Table Michel Vounatsos
Chief Executive Officer (1) | | | |  | | | | Susan H. Alexander Executive Vice President, Chief Legal Officer and Secretary | | | | | | | | | | | | | |  | | | | Jeffrey D. Capello Executive Vice President and Chief Financial Officer | | | |  | | | | Paul F. McKenzie, Ph.D. Executive Vice President, Pharmaceutical Operations & Technology | | | | | | | | | | | | | |  | | | | Michael Ehlers, M.D., Ph.D. Executive Vice President, Research and Development | | | | | | Reflects the potential future payouts of awards granted in 2016 under our annual bonus plan and our LTI program for each NEO as of the grant date. For NEOs hired during 2016 (Mr. Vounatsos and Dr. Ehlers), our annual bonus plan is prorated based on their hire date. |
(2) | Represents the grant date fair value of CSPUs, MSUs, and RSUs, computed in accordance with ASC 718, excluding the effect of estimated forfeitures. These grants were subsequently adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of Bioverativ on February 1, 2017. The amounts reported in this column do not reflect such anti-dilution adjustments. The grant date fair value for MSU grants is estimated as of the date of grant using a lattice model with a Monte Carlo simulation. Assumptions used in this calculation are included on pageF-44 in footnote 15 of our 2016 Annual Report on Form10-K. The grant date fair value for CSPU and RSU grants was determined by multiplying the number of shares subject to the award (assuming target performance for CSPUs) by the closing price of the Company’s common stock on the grant date. |
(3) | These amounts relate to the annual grant of MSUs. These are performance-based RSUs tied to the growth in our stock price between the grant date and each of three annual vesting dates. The number of MSUs earned is determined on each vesting date. Columns (f), (g), and (h) represent the number of MSUs that can be earned based on performance at the threshold level of 50%, target level of 100%, and the maximum level of 200%, respectively. To the extent earned, the award becomes eligible to vest ratably over three years, as described in further detail under the heading “Long-Term Incentives (LTI)” above. |
(4) | These amounts relate to the annual grant of CSPUs. These are performance-based RSUs tied to our 2016 financial performance and subsequently subject to time-based vesting. The number of CSPUs earned is determined in early 2017 based on 2016 revenue and adjusted free cash flow performance against target. Earned CSPUs will vest ratably over three years. These awards are settled in cash or stock at the discretion of our Compensation Committee upon the vesting date based on the30-day average closing price of our common stock. Columns (f), (g), and (h) represent the number of CSPUs earned if the Company performance multiplier were 50%, 100%, and 200%, respectively. |
(5) | These amounts relate to our 2016 annual bonus plan. The amounts shown in column (d) represent the 2016 target payout amount based on the target percentage applied to each NEO’s base salary as of December 31, 2016. For 2016, the bonus targets were 140% of salary for Dr. Scangos and 70% of salary for all other NEOs. In 2016, because the individual performance multiplier was the same as the Company performance multiplier under our 2016 annual bonus plan, the amounts in column (c), (d), and (e) represent a payment if the Company performance multiplier and the individual performance multiplier were each 50%, 100%, and 150%, respectively, which amounts are prorated for Mr. Vounatsos and Dr. Ehlers based on their hire dates. Actual amounts paid to each NEO under this plan are included in the“Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table. |
(6) | These amounts relate to special grants of time-based RSUs, as described in further detail in the CD&A above under the heading “2016 and 2017 Hiring- and Transition-Related Compensation Decisions.” |
| | | | | 47 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued) |
| Outstanding Equity Awards at 2016 FiscalYear-End Executive Summary
|
2018 Highlights We had a productive and successful 2018. We generated record revenues of $13.5 billion for the year, demonstrated resilience in our MS business, continued a strong global launch for SPINRAZA, the first approved treatment for SMA, and made significant progress in our biosimilars business. We added six clinical programs across our strategic core and emerging growth areas and had a strong year for business development. We provided value to our stockholders through the return of approximately $4.4 billion in capital through share repurchases and we continued our leading efforts in environmental, sustainability and diversity matters. Our executive compensation programs for 2018 were aligned with stockholder interests as compensation earned under these programs was closely-linked to the achievement of our corporate performance goals. We achieved or exceeded the vast majority of the corporate performance goals that we set at the beginning of the year under our incentive compensation plans and, accordingly, the payouts under these plans for 2018 were above target payout levels. | | | | | | 31 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
A brief summary of our 2018 business, financial and executive compensation highlights are as follows: Financial Performance The following chart provides a summary of our financial performance for 2018 compared to 2017: 
A reconciliation of our GAAP toNon-GAAP financial measures is provided in Appendix A to this Proxy Statement. Total Stockholder Return Ourone-, three- and five-year total stockholder return (TSR)* compared to our peer group and the Standard & Poor’s 500 (S&P 500) is set forth below. 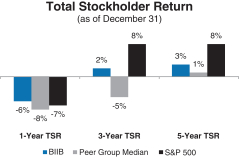
| * | TSR is a measure of performance over time that combines changes in share price and dividends paid to show the total return to the stockholder expressed as an annualized percentage. |
Product and Pipeline Developments The following provides a summary of our product and pipeline developments for 2018: Product Developments In March 2018 we and AbbVie Inc. announced the voluntary worldwide withdrawal of ZINBRYTA for relapsing MS (RMS). In October 2018 we and Samsung Bioepis launched IMRALDI, an adalimumab biosimilar referencing HUMIRA, in Europe. Applications for Marketing and Agency Actions In October 2018 the FDA granted BIIB092, ananti-tau mAb, fast track designation for progressive supranuclear palsy (PSP). In December 2018 Alkermes submitted a NDA to the FDA for the review of BIIB098 (diroximel fumarate). Alkermes is seeking approval of diroximel fumarate under the 505(b)(2) regulatory pathway. If approved, we intend to market diroximel fumarate under the brand name VUMERITY. This name has been conditionally accepted by the FDA and will be confirmed upon approval. | | | | | | 32 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
Clinical Trials MS and Neuroimmunology In September 2018 we completed enrollment of the Phase 2b AFFINITY study evaluating opicinumab, anti-LINGO, as anadd-on therapy in MS patients who are adequately controlled on their anti-inflammatory disease-modifying therapy (DMT), versus the DMT alone. In November 2018 we initiated the Phase 3b NOVA study evaluating the efficacy and safety of extended interval dosing (every six weeks) for natalizumab compared to standard interval dosing in patients with RMS and enrolled the first patient in December 2018. In December 2018 we dosed the first patient in a bioequivalence study to test whether exposure levels of PLEGRIDY are maintained with intramuscular administration. Neuromuscular Disorders | • | | In September 2018 we enrolled the first patient in the Phase 1 study evaluating BIIB078(IONIS-C9Rx), an antisense oligonucleotide (ASO) drug candidate, in adults with C9ORF72-associated ALS. |
| • | | In December 2018 we and our collaboration partner Ionis Pharmaceuticals, Inc. (Ionis) announced results from a positive interim analysis of the ongoing Phase 1 study of BIIB067 (IONIS-SOD1Rx), an investigational treatment for ALS with superoxide dismutase 1 (SOD1) mutations. The following table summarizesinterim analysis showed that, over a three-month period, BIIB067 resulted in a statistically significant lowering of SOD1 protein levels in the equitycerebrospinal fluid and a numerical trend towards slowing of clinical decline as measured by the ALS Functional Rating Scale Revised, both compared to placebo. |
Alzheimer’s Disease and Dementia In May 2018 we initiated a Phase 2 study of BIIB092 for Alzheimer’s disease. In June 2018 we and our collaboration partner Eisai Co., Ltd. (Eisai) announced that elenbecestat, the oral BACE (beta amyloid cleaving enzyme) inhibitor, demonstrated an acceptable safety and tolerability profile in the Phase 2 study, and the results demonstrated a statistically significant difference in amyloid-beta levels in the brain measured byamyloid-PET (positron emission tomography). A numerical slowing of decline in functional clinical scales of a potentially clinically important difference was also observed, although this effect was not statistically significant. In December 2017 we and our collaboration partner Eisai announced that the Phase 2 study of BAN2401, a monoclonal antibody that targets amyloid beta aggregates, an Eisai product candidate for the treatment of Alzheimer’s disease, did not meet the criteria for success based on a Bayesian analysis at 12 months as the primary endpoint in an856-patient Phase 2 clinical study, an endpoint that was designed to enable a potentially more rapid entry into Phase 3 development. In July 2018, based upon the final analysis of the data at 18 months, we and Eisai announced that the topline results from the Phase 2 study demonstrated a statistically significant slowing in clinical decline and reduction of amyloid beta accumulated in the brain. The study achieved statistical significance on key predefined endpoints evaluating efficacy at 18 months on slowing progression in Alzheimer’s Disease Composite Score (ADCOMS) and on reduction of amyloid accumulated in the brain as measured usingamyloid-PET. In July 2018 we completed enrollment of ENGAGE and EMERGE, the Phase 3 studies of aducanumab. In March 2019 we and our collaboration partner Eisai announced that we were discontinuing the EMERGE and ENGAGE Phase 3 studies. Movement Disorders In January 2018 we dosed the first patient in the Phase 2 SPARK study of BIIB054,a-synuclein antibody, in Parkinson’s disease. In September 2018 we completed enrollment of the Phase 2 PASSPORT study of BIIB092 for PSP. Acute Neurology In March 2018 we dosed the first patient in the Phase 2 OPUS study of natalizumab in drug-resistant focal epilepsy. In September 2018 we enrolled the first patient in the Phase 3 CHARM study of BIIB093, glibenclamide IV, in large hemispheric infarction, a severe form of ischemic stroke. | | | | | | 33 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
Neurocognitive Disorders In December 2018 we dosed the first patient in our Phase 2b study of BIIB104 (AMPA) in CIAS. Pain In March 2018 we initiated a Phase 1 study of BIIB095, a Nav 1.7 inhibitor for neuropathic pain. In May 2018 we initiated a Phase 2 study of vixotrigine (BIIB074) in small fiber neuropathy. Other In September 2018 we dosed the first patient in the Phase 2b study of BG00011(STX-100) in idiopathic pulmonary fibrosis, a chronic irreversible and ultimately fatal disease characterized by a progressive decline in lung function. Discontinued Programs In February 2018 we announced that the Phase 2b dose-ranging ACTION study investigating natalizumab in individuals with acute ischemic stroke (AIS) did not meet its primary endpoint. Based on these results, we discontinued development of natalizumab in AIS. The results of the Phase 2b ACTION study do not impact the benefit-risk profile of natalizumab in approved indications, including MS. In October 2018 we announced that we completed the Phase 2b study of vixotrigine (BIIB074) for the treatment of painful lumbosacral radiculopathy (PLSR). The study did not meet its primary or secondary efficacy endpoints and we discontinued development of vixotrigine for the treatment of PLSR. The safety data were consistent with the safety profile reported in previous studies. Business Development In January 2018 we acquired BIIB100 from Karyopharm Therapeutics Inc. BIIB100 is a Phase 1 ready investigational oral compound for the treatment of certain neurological and neurodegenerative diseases, primarily in ALS. BIIB100 is a novel therapeutic candidate that works by inhibiting a protein known as XP01, with the goal of reducing inflammation and neurotoxicity, along with increasing neuroprotective responses. In April 2018 we acquired BIIB104 from Pfizer Inc. BIIB104 is afirst-in-class, Phase 2b ready AMPA receptor potentiator for CIAS, representing our first program in neurocognitive disorders. AMPA receptors mediate fast excitatory synaptic transmission in the central nervous system, a process which can be disrupted in a number of neurological and psychiatric diseases, including schizophrenia. In June 2018 we closed a10-year exclusive agreement with Ionis to develop novel ASO drug candidates for a broad range of neurological diseases (the 2018 Ionis Agreement). We have the option to license therapies arising out of the 2018 Ionis Agreement and will be responsible for the development and potential commercialization of such therapies. In June 2018 we entered into an exclusive option agreement with TMS Co., Ltd. granting us the option to acquireTMS-007, a plasminogen activator with a novel mechanism of action associated with breaking down blood clots, which is in Phase 2 development in Japan, and backup compounds for the treatment of stroke. In June 2018 we exercised our option under our joint venture agreement with Samsung BioLogics to increase our ownership percentage in Samsung Bioepis from approximately 5% to approximately 49.9%. The share purchase transaction was completed in November 2018. In July 2018 we acquired BIIB110 (Phase 1a) andALG-802 (preclinical) from AliveGen Inc. BIIB110 andALG-802 represent novel ways of targeting the myostatin pathway. We initially plan to study BIIB110 in multiple neuromuscular indications, including SMA and ALS. In December 2018 we exercised our option with Ionis and obtained a worldwide, exclusive, royalty-bearing license to develop and commercialize BIIB067, an investigational treatment for ALS with SOD1 mutations. In December 2018 we entered into a collaborative research and license agreement with C4 Therapeutics (C4T) to investigate the use of C4T’s novel protein degradation platform to discover and develop potential new treatments for neurological diseases, such as Alzheimer’s disease and Parkinson’s disease. We will be responsible for the development and potential commercialization of any therapies resulting from this collaboration. | | | | | | 34 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
Share Repurchase Activity In August 2018 our Board of Directors authorized a program to repurchase up to $3.5 billion of our common stock (2018 Share Repurchase Program). Our 2018 Share Repurchase Program does not have an expiration date. All share repurchases under our 2018 Share Repurchase Program will be retired. We returned approximately $4.4 billion to stockholders in 2018 through share repurchases under our 2018 Share Repurchase Program and our 2016 Share Repurchase Program, which was a program authorized by our Board of Directors in July 2016 to repurchase up to $5.0 billion of our common stock and which was completed as of June 30, 2018. Other Notable Achievements in the Workplace and Community Awarded the 2018 International Prix Galien as Best Biotechnology Product for SPINRAZA. The prestigious honor marks the seventh Prix Galien for SPINRAZA, following country recognitions in the U.S., Germany, Italy, Belgium-Luxembourg, the Netherlands and the U.K. The International Prix Galien is given every two years by Prix Galien International Committee members in recognition of excellence in scientific innovation to improve human health. Named the Biotechnology Industry Leader on the Dow Jones Sustainability World Index. Recognized as a corporate sustainability leader with Gold Class and Industry Mover Sustainability Awards from RobecoSAM. Continued commitment to operational carbon neutrality highlighted through the use of 100% renewable electricity globally. Committed to reduce carbon emissions by a targeted amount approved by the Science Based Target Initiative, to align ourselves with the global goal of limiting global temperature rise to under two degrees Celsius. Earned CDP scores of A,A- and B in the areas of Supplier Engagement, Climate Change and Water, respectively. Earned a perfect score of 100% on the Human Rights Campaign’s Corporate Equality Index (a national benchmarking tool on corporate policies and practices pertinent to LGBTQ employees) for the fifth consecutive year. Continued commitment to diversity and inclusion. As of December 31, 2018, 44% of Director-level positions and above were held by women. Over 3,200 employees volunteered from 28 countries during our annual Care Deeply Day. Engaged 50,000+ students inhands-on learning to inspire their passion for science since the inception of Biogen’s Community Labs. 2018 Executive Compensation Programs andPay-for-Performance Alignment We believe our executive compensation programs are effectively designed and have worked well to implement apay-for-performance culture that is aligned with the interests of our stockholders. In 2018 our executive compensation programs consisted of base salary, short- and long-term incentives and other benefits. 91% of our CEO’s and 84% of our other NEOs’ 2018 target compensation was performance-based andat-risk. 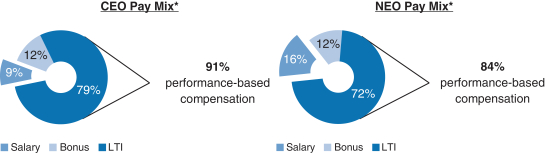
| * | Reflects annual salary, target bonus and target grant value of the 2018 annual long-term incentive awards. The NEO compensation mix excludes theone-time transition awards thatof RSUs granted to Dr. Ehlers, Ms. Alexander and Dr. McKenzie, as described in further detail below. |
| | | | | | 35 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
100% of our NEOs’ 2018 annual long-term incentive (LTI) grants were performance-based andat-risk. | | | 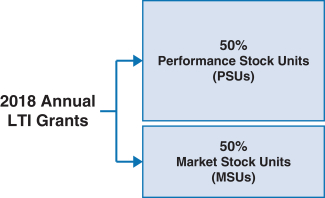 | | • 60% earned based on achievement of three-year adjustedNon-GAAP diluted earnings per share (EPS) and pipeline milestone performance goals • 40% earned based on achievement of adjustedNon-GAAP free cash flows and revenues over threeone-year performance periods • PSUs were outstandingintroduced in 2018. For more information on our PSUs, please see “Long-Term Incentives – 2018 PSUs” below. • Earned based on stock price performance over one, two and three year periods |
Our 2018 performance-based compensation payouts align with our commitment to strong performance. In 2018 we exceeded the vast majority of the corporate performance goals that we set at the beginning of the year for our incentive compensation plans. As a result, the payouts, as a percentage of target, for our 2018 annual bonus plan and the portions of our PSUs and MSUs that were eligible to be earned based on 2018 performance were above target payout amounts, as described in further detail below. 2018 Advisory Vote on Executive Compensation | | | At our 2018 annual meeting of December 31, 2016stockholders, we continued to receive strong support for eachour executive compensation programs with approximately 95% of the votes cast for approval of our NEOs.annual“say-on-pay” proposal. Our C&MD Committee viewed this as positive support for our executive compensation programs and their alignment with long-term stockholder value creation and determined that the Company’s executive compensation programs have been effective in implementing the Company’s stated compensation philosophy and objectives. | | 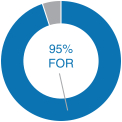
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (a) | | | | | Option Awards(1) | | | Stock Awards | | | | | | | | | | | | | | | | | | | | | | | | | Equity Incentive Plan
Awards | | | | | | | | | | | | | | | | Option
Expiration
Date (f) | | | Number
of
Shares
or Units
of Stock
That
Have
Not
Vested(2) (g) | | | Market
Value of
Shares or
Units of
Stock
That
Have Not
Vested(3) (h) | | | Number
of
Unearned
Shares or
Units
That
Have Not
Vested(4)
(i) | | | Market
Value of
Unearned
Shares or
Units That
Have Not
Vested(3)
(j) | | | | Grant
Date (b) | | | Number of Securities
Underlying Unexercised
Options | | | Option
Exercise
Price (e) | | | | | | | | | | Exercisable (c) | | | Unexercisable (d) | | | | | | | | | George A. Scangos | | | 2/12/2013 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 9,191 | | | $ | 2,606,384 | | | | | 2/12/2014 | | | | — | | | | — | | | | — | | | | — | | | | 10,656 | | | $ | 3,021,828 | | | | — | | | | — | | | | | 2/12/2014 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,251 | | | $ | 1,489,079 | | | | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | 8,397 | | | $ | 2,381,221 | | | | — | | | | — | | | | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 8,851 | | | $ | 2,509,967 | | | | | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | 28,890 | | | $ | 8,192,626 | | | | — | | | | — | | | | | | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 40,460 | | | $ | 11,473,647 | | | Michel P. Vounatsos | | | 5/2/2016 | | | | — | | | | — | | | | — | | | | — | | | | 6,798 | | | $ | 1,927,777 | | | | — | | | | — | | | | | | 5/2/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 9,520 | | | $ | 2,699,682 | | | Paul J. Clancy | | | 2/12/2013 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,808 | | | $ | 796,293 | | | | | 2/12/2014 | | | | — | | | | — | | | | — | | | | — | | | | 2,483 | | | $ | 704,129 | | | | — | | | | — | | | | | 2/12/2014 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,224 | | | $ | 347,102 | | | | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | 1,641 | | | $ | 465,355 | | | | — | | | | — | | | | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,731 | | | $ | 490,877 | | | | | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | 7,902 | | | $ | 2,240,849 | | | | — | | | | — | | | | | | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 11,060 | | | $ | 3,136,395 | | | John G. Cox | | | 2/12/2008 | | | | 2,892 | | | | — | | | $ | 60.56 | | | | 2/11/2018 | | | | — | | | | — | | | | — | | | | — | | | | | 2/24/2009 | | | | 7,588 | | | | — | | | $ | 49.65 | | | | 2/23/2019 | | | | — | | | | — | | | | — | | | | — | | | | | 2/12/2013 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,249 | | | $ | 637,771 | | | | | 2/12/2014 | | | | — | | | | — | | | | — | | | | — | | | | 2,483 | | | $ | 704,129 | | | | — | | | | — | | | | | 2/12/2014 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,224 | | | $ | 347,102 | | | | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | 2,297 | | | $ | 651,383 | | | | — | | | | — | | | | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,421 | | | $ | 686,547 | | | | | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | 8,466 | | | $ | 2,400,788 | | | | — | | | | — | | | | | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 11,850 | | | $ | 3,360,423 | | | | | | 4/1/2016 | | | | — | | | | — | | | | — | | | | — | | | | 8,444 | | | $ | 2,394,550 | | | | — | | | | — | | | Michael D. Ehlers | | | 6/1/2016 | | | | — | | | | — | | | | — | | | | — | | | | 8,266 | | | $ | 2,344,072 | | | | — | | | | — | | | | | | 6/1/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 7,320 | | | $ | 2,075,806 | |
| Notes
Our C&MD Committee is committed to continually reviewing our executive compensation programs on a proactive basis to ensure the ongoing alignment of such programs with the interests of our stockholders. | In 2018 our C&MD Committee reviewed the external landscape, the results from our“say-on-pay” proposal at last year’s annual meeting of stockholders and the Company’s performance against the current compensation programs. Our C&MD Committee was satisfied that our existing compensation programs further ourpay-for-performance philosophy, but made certain enhancements to the Outstanding Equity Awards at 2016 Fiscal Year End Tabledesign of our LTI program in 2018 to strengthen its focus on long-term performance and alignment with our stockholders’ interests. |
Specifically, under our 2018 LTI program, grants of PSUs replaced grants of cash-settled performance units (CSPUs), which we had granted in previous years. The key changes are as follows: (1) | All stock options were granted with aten-year term. Stock options vest 25% on each of the first four anniversaries of the grant date. It has not been the Company’s practice to cash out stock options having an exercise price greater than the market price (i.e., underwater options). These stock options were subsequently adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of Bioverativ on February 1, 2017. The amounts reported in this column do not reflect such anti-dilution adjustments.PSU awards are subject to three-year cliff vesting as compared to annual ratable vesting over three years (1/3 per year) for CSPU awards; 60% of PSU awards are earned over a three-year performance period based on the achievement of three-year cumulative performance goals for stock-settled PSU awards and 40% of PSU awards are earned over three annual performance periods based on the achievement of three sets of annual performance goals for cash-settled PSU awards as compared to 100% of CSPUs awards earned based upon one annual performance period for CSPU awards; and 60% of the PSU awards will be settled in stock and 40% of the PSU awards will be settled in cash as compared to 100% cash settlement for CSPU awards. | | | | | | 36 | |  | |  |
(2) | CSPUs were granted in 2016, 2015, and 2014. Numbers reflect the number of CSPUs earned and eligible to vest based on our financial performance for each of 2016, 2015, and 2014, but that have not satisfied the service-based vesting requirement as of December 31, 2016. CSPUs that have been earned upon satisfaction of the performance conditions vest ratably over three years from the grant date. The cash payout for these awards will be based on the30-day average closing stock price at vesting. For Mr. Cox and Dr. Ehlers, the amounts in this column also reflect 8,444 RSUs granted to Mr. Cox under his special recognition award on April 1, 2016 and 3,034 RSUs granted to Dr. Ehlers on June 1, 2016 in connection with his hire, each vesting ratably over three years from the grant date. These grants were subsequently adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of Bioverativ on February 1, 2017. The amounts reported in this column do not reflect such anti-dilution adjustments.
| | | | 5 | | Executive Compensation Matters (continued) |
(3) | The market value of awards is based on the closing price of our stock on December 30, 2016 ($283.58), the last business day of 2016, as reported by NASDAQ. |
(4) | MSUs were granted in 2016, 2015, 2014, and 2013. These are performance-based RSUs tied to the growth in our stock price between the dates of grant and vesting. MSUs are eligible to vest ratably over four years for grants made in 2013, and three years for grants made in 2014, 2015, and 2016. The number and value shown in columns (i) and (j), respectively, reflects maximum performance results for MSUs granted in 2013 and 2016 and target performance results for MSUs granted in 2014 and 2015 based on the prior year’s performance in each case. These grants were subsequently adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of Bioverativ on February 1, 2017. The amounts reported in this column do not reflect such anti-dilution adjustments. |
2016 Option Exercises and Stock VestedFor additional information on our PSU awards, please see “Long-Term Incentives – 2018 PSUs” below. Roles and Responsibilities Role of our C&MD Committee Our C&MD Committee, which is composed of four independent directors, oversees and administers our executive compensation programs. In making executive compensation decisions, our C&MD Committee reviews a variety of factors and data, most importantly our performance and individual executives’ performance, and considers the totality of compensation that may be paid. In addition, our C&MD Committee administers our annual bonus plan and our equity plans, reviews business achievements relevant to payouts under our compensation plans, makes recommendations to our Board of Directors with respect to compensation policies and practices as well as the compensation of our CEO and seeks to ensure that total compensation paid to our executive officers is fair, competitive and aligned with stockholder interests. Our C&MD Committee retains the right to hire outside advisors as needed to assist it in reviewing and revising our executive compensation programs. The duties and responsibilities of our C&MD Committee are described on page 20 and can be found in our C&MD Committee’s written charter adopted by our Board of Directors, which can be found on our website,www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the website. Role of the Independent Compensation Consultant Our C&MD Committee believes that independent advice is important in developing and overseeing our executive compensation programs. Frederic W. Cook & Co., Inc. (FW Cook) served as our C&MD Committee’s independent compensation consultant until June 2018 and advised our C&MD Committee regarding compensation decisions in 2018. FW Cook did not provide any other services to Biogen. Pearl Meyer & Partners LLC (Pearl Meyer) has served as our C&MD Committee’s independent compensation consultant since June 2018 and has advised our C&MD Committee regarding compensation decisions since that time. Pearl Meyer does not provide any other services to Biogen and engages in other matters as needed and as directed solely by our C&MD Committee. References in this CD&A to our independent compensation consultant refer to FW Cook for the period during which it was engaged and to Pearl Meyer thereafter. Reporting directly to our C&MD Committee, our independent compensation consultant provides guidance on trends in CEO, executive andnon-employee director compensation, the development of specific executive compensation programs and the composition of the Company’s compensation peer group. Additionally, our independent compensation consultant prepares a report on CEO pay that compares each element of compensation to that of CEOs in comparable positions at companies in our peer group. Using this and other similar information, our C&MD Committee recommends, and our Board of Directors approves, the elements and target levels of our CEO’s compensation. During 2018 the Company paid FW Cook and Pearl Meyer $123,275 and $47,666, respectively, in consulting fees directly related to these services. Our C&MD Committee assessed FW Cook’s independence annually and, in accordance with applicable SEC and Nasdaq rules, confirmed in December 2017 that FW Cook’s work did not raise any conflicts of interest and that FW Cook remained independent under applicable rules. Our C&MD Committee assessed Pearl Meyer’s independence in connection with its engagement in June 2018 and, in accordance with applicable SEC and Nasdaq rules, confirmed in December 2018 that Pearl Meyer’s work did not raise any conflicts of interest and that Pearl Meyer remains independent under applicable rules. Role of our CEO Each year our CEO provides an assessment of the performance of each executive officer, other than himself, during the prior year and recommends to our C&MD Committee the compensation to be paid or awarded to each executive. Our CEO’s recommendations are based on numerous factors, including: Company, team and individual performance; potential for future contributions; leadership competencies; external market competitiveness; internal pay comparisons; and other factors deemed relevant. To understand the external market competitiveness of the compensation for our executive officers, our CEO and our C&MD Committee review a report analyzing publicly-available information and surveys prepared by our internal | | | | | | 37 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
compensation group and reviewed by our independent compensation consultant. The report compares the compensation of each executive officer, other than our CEO, to data for comparable positions at companies in our peer group, by compensation element (please see “External Market Competitiveness and Peer Group” below for further details). Our C&MD Committee considers all of the information presented, discusses the recommendations with our CEO and with our independent compensation consultant and applies its judgment to determine the elements of compensation and target compensation levels for each executive officer other than the CEO. Our CEO also provides a self-assessment of his achievements for the prior year. Our C&MD Committee reviews and considers this in analyzing the CEO’s performance, and in recommending for approval by our Board of Directors, the compensation of our CEO. Our CEO does not participate in any deliberations regarding his own compensation. Executive Compensation Philosophy and Objectives Our executive compensation programs are designed to drive the creation of long-term stockholder value by delivering performance-based compensation that is competitive with our peer group in order to attract and retain extraordinary leaders who can perform at high levels and succeed in a demanding business environment. We aim to achieve this by designing programs that are: | • | | Mission Focused and Business Driven.Our executive officers must usecompensation programs support the relentless pursuit of delivering meaningful and innovative therapies to patients by providing our executives with incentives to achieve the near- and long-term objectives of our business. Substantially all of our executive incentive compensation programs are tied directly, and meaningfully, to Company performance. Our objective is to emphasize the importance of achieving short-term goals while building and sustaining a foundation for long-term success. |
| • | | pre-establishedCompetitively Advantageous. trading plansWe benchmark our executive compensation programs against a peer group of biotechnology and pharmaceutical companies that we believe are representative of the companies we primarily compete with for talent, balanced with factors such as business scope and size, including revenues and market capitalization, business focus and geographic scope of operations. Peer group practices are among the many factors we take into account in developing compensation programs that we believe are most effective, and which |
| | enable us to sell sharesrecruit, retain and motivate our leadership team to achieve their best for Biogen and our stockholders. |
| • | | Performance Differentiated. We believe strongly inpay-for-performance and endeavor to significantly differentiate rewards by delivering the highest rewards to our best performers and lesser rewards to those who do not meet our performance expectations. |
| • | | Ownership Aligned. At Biogen, we believe every employee contributes to the success of Biogen stock. Trading plans may only be entered into when the executive is not in possession of materialnon-public information about the Company and, we require | | | | | 48 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued) |
as such, every employee has a waiting period following the establishment of a trading plan before any trades may be executed. Our policy is designed to provide safeguards that will allow our executives an opportunity to realize the value intended by the Company in granting equity-based LTI awards. Our NEOs are also subject to the share ownership guidelines described abovevested interest in the subsection titled “Share Ownership Guidelines.”
The following table shows information regarding vestingCompany’s success. To reinforce this alignment with our stockholders, we strongly encourage stock ownership through our equity-based compensation programs. For members of stock awards for each NEO duringour executive team, including our NEOs, who set and lead the year ended December 31, 2016. Nonefuture strategic direction of our Company, we ensure that a significant portion of their total pay opportunities are equity-based to maintain alignment between the NEOs exercised stock options during the year ended December 31, 2016.
| | | | | | | | | | | | | | | | Stock Awards | | | Name | | Number of Shares
Acquired on
Vesting(1) | | | | | | Value
Realized on
Vesting(2)(3) | | George A. Scangos | | | 57,609 | | | | | | | $ | 14,815,081 | | Paul J. Clancy | | | 15,242 | | | | | | | $ | 3,976,993 | | John G. Cox | | | 15,205 | | | | | | | $ | 3,950,976 | |
Notes to the 2016 Option Exercises and Stock Vested Table
(1) | With the exceptioninterests of Dr. Scangos’ 2014 CSPUs, CSPUs were settled in cash for all of our NEOs. The number of actual shares of our common stock acquired on vesting after shares were withheld to pay the minimum withholding of taxes was as follows: |
| | | | | Net Shares
Acquired(4)
| Dr. Scangos
| | 20,823 | Mr. Clancy
| | 4,613 | Mr. Cox
| | 4,876 |
(2) | The value realized for MSUs and RSUs are calculated by multiplying the closing price of a share of our common stock on the vesting date by the total number of shares that vested on such date. The value realized for CSPUs is calculated using the60-day average closing price of the common stock of the Company through the vesting date for grants made prior to 2014 and the30-day average closing price for grants made in 2014 and later. |
(3) | The value realized upon vesting for Mr. Cox includesnon-qualified deferred CSPUs of $928,065. Terms of thenon-qualified deferred compensation plan are presented in the narrative preceding the 2016Non-Qualified Deferred Compensation Table below. |
(4) | MSUs were settled in shares of our common stock. CSPUs were settled in cash for all of our NEOs, other than Dr. Scangos, in which case a portion of his CSPUs were settled in shares of our common stock. For Dr. Scangos, in 2015, our Compensation Committee exercised its discretion to settle Dr. Scangos’ 2014 CSPUs in shares of our common stock; the net shares acquired by Dr. Scangos reflected in the table above represent 14,792 MSUs and 6,031 CSPUs settled in shares. |
2016Non-Qualified Deferred Compensation
The SSP covers our executive officers and other management employeesour stockholders.
|
| • | | Flexible. We are committed to providing flexible benefits designed to allow our diverse global workforce to have reward opportunities that meet their varied needs so that they are inspired to perform their very best on behalf of patients and stockholders each day. |
External Market Competitiveness and Peer Group We consider market practices and trends when determining executive compensation levels and compensation program designs at Biogen. We do not target a specific market percentile or simply replicate the market practice. Instead, we review external market practices as a reference point to assist us in providing programs designed to attract, retain and inspire extraordinary talent. Our C&MD Committee also uses a peer group to provide context for its executive compensation decision-making. Each year our independent compensation consultant reviews the external market landscape and evaluates the composition of our peer group for appropriateness. Our C&MD Committee reviews the information provided from internal sources as well as the information provided by our independent compensation consultant to select our peer group based on comparable companies that approximate (1) our scope of business, including revenues and market capitalization, (2) our global geographical reach, (3) our research-based business with multiple marketed products and (4) a comparable pool of talent for which we compete. The peer group for determining our 2018 compensation decisions consisted of biotechnology and pharmaceutical | | | | | | 38 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
companies, as we compete with companies in both of these sectors for executive talent. | | | Biotechnology Peers | Alexion Pharmaceuticals, Inc. Amgen Inc. Celgene Corporation Gilead Sciences Inc. Vertex Pharmaceuticals International, Inc. | | | Pharmaceutical Peers | AbbVie Inc. Allergan plc Bristol-Myers Squibb Company Eli Lilly and Company Merck & Co, Inc. Mylan N.V. Bausch Health Companies (f/k/a Valeant Pharmaceuticals Incorporated) |
For each of the companies in our peer group, where available, we analyze the company’s Compensation Discussion and Analysis and other data publicly filed during the prior year to identify the executives at such companies whose positions are comparable to those held by our executive officers. We then compile and analyze the data for each comparable position. Our competitive analysis includes the structure and design of the compensation programs as well as the targeted value of the compensation under these programs. For our executive officers other than our CEO, we may supplement the data from our peer group with published compensation surveys where appropriate. For 2018, consistent with past years, we used theWillisTowersWatson U.S. CDB Pharmaceutical and Health Sciences Executive Compensation Database survey (which we refer to as the Willis Towers Watson survey). We chose the Willis Towers Watson survey because of the number of companies in our peer group that participate in it, the number of positions reported by the survey that continue to be comparable to our executive positions and the high standards under which we understand the survey is conducted (including data collection and analysis methodologies). All of the companies in our peer group are represented in a special cross-section of the Willis Towers Watson survey focused on our peer group, other than Bausch Health Companies (formally known as Valeant Pharmaceuticals Incorporated), which did not participate in the survey. Compensation Elements Our C&MD Committee determines the elements of compensation we provide to our executive officers. The elements of our executive compensation programs and their objectives are as follows: | | | | | | | | | | | | Element | | | | Objective(s) | | | Base Salary | | • | | Provides a fixed level of compensation that is competitive with the external market and reflects each executive’s contributions, experience, responsibilities and potential to contribute to our future success. | | | Annual Bonus Plan | | • | | Aligns short-term compensation with the annual goals of the Company. | | | | • | | Motivates and rewards the achievement of annual Company and individual performance goals that support short- and long-term value creation. | | | Long-term Incentives | | • | | Aligns executives’ interests with the long-term interests of our stockholders by linking the value of awards to increases in our stock price. | | | | | • | | Motivates and rewards the achievement of stock price growth andpre-established corporate performance goals, including those with a longer-term focus. | | | | | • | | Promotes executive retention and stock ownership and focuses executives on enhancing long-term stockholder value. | | | Benefits | | • | | Promotes health and wellness. | | | | | • | | Provides financial protection in the U.S. Employees whose base salaryevent of disability or death. | | | | | • | | Providestax-beneficial ways for executives to save towards their retirement and annual cash incentivesencourages savings through competitive matches to executives’ retirement savings. | | |
Compensation Mix Our C&MD Committee determines the general mix of the elements of our executive compensation programs. It does not target a specific mix of value for the compensation elements within these programs in either the program design or pay decisions. Rather, our C&MD Committee reviews the mix of compensation elements to ensure an appropriate level of performance-based compensation is apportioned to the short-term and even more to the long-term to ensure alignment with our business goals and performance. Additionally, our C&MD Committee believes the greater the leadership responsibilities, the greater the potential impact an individual will have on Biogen’s future strategic direction. Therefore, for our executive officers, including our NEOs, additional emphasis is placed on performance-based compensation, with a particular emphasis on LTI. The 2018 compensation mix for Mr. Vounatsos and our other NEOs was highly performance-based andat-risk; 91% of 2018 compensation was performance-based for Mr. Vounatsos and 84% of 2018 compensation was | | | | | | 39 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
performance-based for our other NEOs, assuming target level achievement of applicable corporate performance goals and with LTI awards measured at target grant date values, and excluding theone-time transition awards of RSUs granted to Dr. Ehlers, Ms. Alexander and Dr. McKenzie, as described in further detail below. Performance Goals and Target Setting Process Early each year, our C&MD Committee reviews and establishes the pay levels of each element of total compensation for our executive officers. Total compensation is comprised of base salary, annual bonus and LTI awards. As part of this process, our C&MD Committee reviews the mix of compensation elements to ensure our performance-based compensation is apportioned appropriately and aligns with our business goals and performance. Our C&MD Committee also ensures that the performance metrics and goals are aligned with the annual business plan approved by our Board of Directors so there is full alignment of executive incentive goals with the goals that have been established for the year. Executive officers are also evaluated based on qualitative factors, such as individual, strategic and leadership achievements. The use of both quantitative and qualitative metrics, as well as the weighting of such metrics, effectively mitigates the impact of a single risk, such as dependence on drug pricing, pipeline performance or market share, on overall compensation. | | | | | | 40 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
A summary of the process our C&MD Committee follows in setting compensation is described below: | | | | |  Target Setting Target Setting
| | 
 Monitoring & Tracking Monitoring & Tracking
• Our C&MD Committee closely monitors progress against the performance goals throughout the year exceed a specified limit ($265,000and engages in 2016) receive a Company-paid restoration matchdialogue with management on such progress. | |  Results & Awards: Results & Awards:
C&MD Committee Actions
• Reviews and discusses the portionperformance of our executive officers against their respective performance goals. • Reviews and discusses the Company, team and individual performance of each executive officer, other than our CEO, as assessed by our CEO. • Reviews and discusses our CEO’s recommended compensation levels for each executive officer, other than himself, in the context of such executive officer’s contributions to the Company and the other factors described above. • Approves the final compensation for each executive officer other than our CEO, including base salary, annual bonus and payments in respectLTI awards. • Reviews CEO compensation and recommends to our Board of CSPUs that exceeds this limit;Directors for approval the restoration match equals 6%compensation of this excess compensation. The restoration match feature is intended to replace the amount of matching employer contributions that the participant would otherwise have been eligible to receive under our 401(k) plan but for the $265,000 limit imposed by Section 401(a)(17) of the Internal Revenue Code. In addition, eligible employees may make voluntary contributions of up to 80% of theirCEO, including base salary, and 100% of their annual bonus and cash payments in respect of CSPUsLTI awards. | • Our C&MD Committee and our CEO discuss potential goals for the upcoming year that are tied to the SSP,short- and thereby defer income taxes on such amounts until distribution is made from the SSP. The Company does not match participants’ voluntary contributions to the SSP. The SSP provides for immediate vesting of the restoration match consistent with our immediate vesting longer-term strategic goals of the Company match provided under our 401(k) plan.
Notional SSP accounts are maintained for each participant. Accounts include employee and employer contributions and reflect the performance of notional investments selected by the employee or a default investment if the employee does not make a selection. These notional investment options include the mutual funds offered under our 401(k) plan as well as individual goals for our executive officers.
• The annual business plan for the year is approved by our Board of Directors. As part of the approval process, our Board considers many factors relevant to our business, reputation and strategy, including pipeline and business development, pricing and patient access, market expectations and intellectual property risk. • Our C&MD Committee ensures that the performance goals and targets under our compensation plans are aligned with the approved annual business plan. • Payout levels for each performance goal are established by management and approved by our C&MD Committee. • The performance goals are then applied to the compensation opportunities for our executive officers, including NEOs, so that there is full alignment of executive incentive goals with the goals that have been established for the year. • Our C&MD Committee also reviews base salaries, bonus and LTI planning ranges, plan designs, benefits and peer group data. |
| | | | | | 41 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
2018 Base Salary Our Board of Directors reviewed the base salaries of chief executive officers in our peer group and considered Mr. Vounatsos’ compensation mix, capabilities, performance and future expected contributions. Mr. Vounatsos’ base salary was set at $1,300,000, which positioned him below the market median when compared to the chief executive officers of our peer group. Our C&MD Committee undertook a similar review when approving the base salaries for our other NEOs, which positioned them, on average, slightly below the market median compared to persons with comparable jobs within our peer group. The annual base salary of each of our NEOs in 2018, compared to 2017, was as follows: | | | | | | | | | | | | | | Name | | 2018 Salary | | | 2017 Salary | | | % Increase(1) | | | M. Vounatsos | | $ | 1,300,000 | | | $ | 1,100,000 | | | 18.2% | | | J. Capello(2) | | $ | 750,000 | | | $ | 750,000 | | | n/a | | | M. Ehlers | | $ | 834,094 | | | $ | 794,375 | | | 5.0% | | | S. Alexander | | $ | 749,177 | | | $ | 723,842 | | | 3.5% | | | P. McKenzie | | $ | 633,938 | | | $ | 603,750 | | | 5.0% | | |
| (1) | Percentage increase reflects the annual merit increase and, in the case of Mr. Vounatsos, also includes a fixed rate option which earns a ratemarket adjustment. |
| (2) | Mr. Capello was hired in November 2017. The initial determination of returnhis base salary took into account the Company’s peer group data. |
2018 Performance-Based Plans and Goal Setting Our executive compensation programs place a heavy emphasis on performance-based compensation. We maintain a short-term incentive plan, known as our annual bonus plan, as well as an LTI plan. Awards to our NEOs under our annual bonus plan have been made under our 2008 Performance-Based Management Incentive Plan, and awards under our LTI plan are granted under our 2017 Omnibus Equity Plan. Awards made under our annual bonus plan are directly tied to the achievement of our corporate performance goals, which are aligned with the Company’s short- and long-term strategic plans, as well as individual performance goals. Awards made under our LTI plan are directly tied to the performance of the price of our common stock, which aligns our executives’ long-term interests with the interests of our stockholders. A portion of our LTI awards are also tied to the Company’s financial performance, as described below under “Long-Term Incentives – 2018 PSUs.” In setting our annual goals under our short- and long-term incentive plans, in addition to our internal forecasts, we consider analysts’ projections for our performance and the performance of companies in our peer group, as well as broad economic and industry trends. We strive to establish challenging targets that result in payouts at or above target levels only when Company performance warrants it. Our C&MD Committee is responsible for reviewing and approving our annual goals, targets and levels of payout (e.g., threshold, target and maximum) for our executive incentive compensation plans and for reviewing and determining actual performance results at the end of the applicable performance period. In setting and approving the corporate performance goals for our executive officers and for the Company under both the short- and long-term incentive plans, our C&MD Committee also considers the alignment of such goals to our business plan, the degree of difficulty of attainment and the potential for the goals to encourage inappropriate risk-taking. Our C&MD Committee has determined that the structures of our executive compensation programs do not put our patients, investors or the Company at any material risk. Annual Bonus Plan Our annual bonus plan is a cash incentive plan that rewards near-term financial, strategic and operational performance. Our C&MD Committee reviews the annual target bonus opportunities for each executive officer by position each year to ensure such opportunities remain competitive. No significant changes were made in 2018 to the target annual bonus opportunities, as a percentage ofyear-end annual base salary, for any of our NEOs other than Mr. Vounatsos, whose target annual bonus opportunity was market adjusted and increased from 125% of base salary in 2017 to 140% of base salary in 2018. In accordance with our policy, target annual bonus opportunities for all of our other NEOs in 2018 were determined based on their positions as Executive Vice Presidents. | | | | | | 42 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
The target annual bonus opportunity as a percentage ofyear-end annual base salary each of our NEOs in 2018 compared to 2017 was as follows: | | | | | | | | | | Name | | 2018 Target | | | 2017 Target | | M. Vounatsos | | | 140% | | | | 125% | | J. Capello | | | 70% | | | | 70% | | M. Ehlers | | | 70% | | | | 70% | | S. Alexander | | | 70% | | | | 70% | | P. McKenzie | | | 70% | | | | 70% | |
2018 Annual Bonus Plan Design Awards for our NEOs under our 2018 annual bonus plan were based on the achievement of Company performance goals and individual performance goals. At the beginning of 2018, our C&MD Committee set multiple Company performance goals for our 2018 annual bonus plan and provided for a payout multiplier, which we refer to as the Company Multiplier, ranging from 0% to 150%, for each Company goal based on the determination of the level of achievement of each goal and application of the weighting assigned to each goal, which determined the Company Multiplier applied to the bonus calculation. The Company Multiplier ranged from 0% to 150% as follows: | | | | | | | | | | | | | | Performance Multipliers | | Below Threshold | | Threshold | | Target | | Max | Company | | 0% | | 50% | | 100% | | 150% |
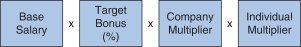
In addition, our 2018 annual bonus plan payouts were also based on an assessment of each NEO’s individual performance, taking into account his or her achievement of individual performance goals. Evaluating individual performance allows our C&MD Committee the discretion to increase or decrease each NEO’s bonus amount based on the NEO’s performance by applying an individual performance multiplier, ranging from 0% to 150%, which we refer to as the Individual Multiplier. We determined the individual annual bonus payments for 2018 using the following calculation: 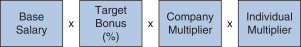
Our 2018 annual bonus plan provided that if the Company Multiplier was less than 50%, there would be no payout, regardless of individual performance, further strengthening ourpay-for-performance philosophy. Further, because the Individual Multiplier and the Company Multiplier each have a maximum of 150%, the combined multiplier result for each NEO could not exceed 225%. 2018 Company Performance Goals and Results Company performance goals were established at the start of 2018 with assigned weightings that reflected the Company’s focus on attaining both financial and strategic goals (pipeline performance, MS leadership, continued SMA launch excellence and enhancing our strategic alliances). The goals and weightings we selected reflect the importance of linking reward opportunities to both near-term results and our progress in achieving longer-term goals. The strategic goals we selected in 2018 were designed to measure the achievement of our annual strategic priorities relating to our commercial opportunities and pipeline progress. Our financial performance goals were based on the Company’s annual operating plan and long-range plan approved by our Board of Directors and with reference to analyst consensus for Biogen revenues andNon-GAAP diluted EPS based on the most current analyst reports at the time we set our targets. The following table presents our financial targets relative to analysts’ consensus for 2018: 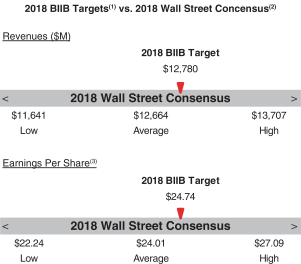
(1) Please see “2018 Annual Bonus Plan Company Performance Targets and Results Table” below for more details. (2) Wall Street figures reflect estimates made in January 2018 for the Biogen fiscal year ending December 31, 2018. (3) ReflectsNon-GAAP diluted EPS. | | | | | | 43 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
2018 Annual Bonus Plan Company Performance Targets and Results Table Set forth below is a summary of the Company performance goals and weightings that our C&MD Committee established for our 2018 annual bonus plan and the degree to which we attained these Company performance goals. As described below, the Company Multiplier for the 2018 Annual Bonus Plan was 131%, reflecting the strong performance relative to ourpre-established goals. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Performance Range | | | | | | | | | | | | | | | Company Goals | | Weight | | | Threshold | | | Target | | | Max | | | Results | | | Company Multiplier | | FINANCIAL PERFORMANCE | | | | | | | | | | | | | | | | | | | | | | | | | Revenues | | | 20 | % | | $ | 12,310M | | | $ | 12,780M | | | $ | 13,250M | | | $ | 13,363M | (1) | | | 150.0 | % | Non-GAAP diluted EPS | | | 20 | % | | $ | 23.47 | | | $ | 24.74 | | | $ | 26.01 | | | $ | 26.89 | (1) | | | 150.0 | % | MARKET PERFORMANCE | | | | | | | | | | | | | | | | | Achieve Global MS Market Share | | | 15 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Below
Goal(2) |
| | | 91.8 | % | MS Leader in Customer Trust and Value Survey | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(2) |
| | | 125.0 | % | Achieve Global SMA Market Share | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(2) |
| | | 134.9 | % | PIPELINE DEVELOPMENT | | | | | | | | | | | | | | | | | Build and Advance Total Pipeline | | | 10 | % | |
| Specific pipeline goals
are not disclosed for competitive reasons |
| |
| Above
Goal(3) |
| | | 110.0 | % | Achieve Aducanumab Phase 3 Enrollment | | | 5 | % | |
| Specific enrollment goals
are not disclosed for competitive reasons |
| |
| Above
Goal(4) |
| | | 105.0 | % | COLLABORATION | | | | | | | | | | | | | | | | | Improve and Expand Key Strategic Alliances | | | 10 | % | |
| Specific strategic
alliance goals are not
disclosed for competitive reasons |
| |
| Above
Goal(5) |
| | | 150.0 | % | Company Multiplier | | | | 131.0 | %* |
| * | Numbers may not recalculate due to rounding. |
Notes to 2018 Annual Bonus Plan Company Performance Targets and Results Table | (1) | These financial measures were based on our publicly reported revenues of $13,453 million and our publicly announcedNon-GAAP diluted EPS of $26.20, as adjusted as follows: for purposes of our 2018 annual bonus plan, revenues andNon-GAAP diluted EPS were adjusted to neutralize the effects of foreign exchange rate fluctuations.Non-GAAP diluted EPS was further adjusted to add back $1.21 to reflect the impact of additional research and development expense recognized in 2018 resulting from the 2018 Ionis Agreement and $0.07 to neutralize the unfavorable impact of the worldwide withdrawal of ZINBRYTA, partially offset by the Company’s retirement committee. For contributionssubtraction of $0.59 related to higher than originally contemplated stock repurchases in 2018, as these charges were not originally contemplated at the time the Company performance goals were determined. |
| (2) | Achievement of market goals for MS was below goal and achievement of MS leader and market goals for SMA were above goals. Specific details are not disclosed for competitive reasons. |
| (3) | The Company continued to expand andre-shape its pipeline ofpre-clinical and clinical stage programs through the advancement of internal programs, external business development activities and exceeding expectations with respect to the SSP fixed rate optionlevel of confidence in 2016, this rateand momentum of return is set at 5.50%. Contributions to the fixedits clinical stage portfolio. Specific details are not disclosed for competitive reasons. |
| (4) | Aducanumab Phase 3 clinical trial patient enrollment was above goal. Specific details are not disclosed for competitive reasons. |
| (5) | Key strategic alliance and acquisition activities were above goal. Specific details are not disclosed for competitive reasons. |
| | | | | | 44 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
2018 Individual Performance Goals and Results The Individual Multiplier reflects each named executive officer’s overall individual performance rating as part of our performance assessment process. Unlike our formulaic calculation of corporate performance against Company performance goals in determining the Company Multiplier, each named executive officer’s Individual Multiplier is based on a subjective evaluation of his or her overall performance and consideration of the achievement of individual goals established at the beginning of the year. Goals may be both quantitative and qualitative. For 2018 Mr. Vounatsos recommended to our C&MD Committee an Individual Multiplier for each named executive officer other than himself based on his assessment of their individual contributions for the full year. Our C&MD Committee considered all of the information presented, discussed our CEO’s recommendations with him and its independent compensation consultant and applied its judgment to determine the Individual Multiplier for each named executive officer. Our Board of Directors determined Mr. Vounatsos’ Individual Multiplier based on its assessment of his performance. In its evaluation, our C&MD Committee assigned Individual Multipliers to our named executive officers of between 115% and 140% based on the following accomplishments during 2018: Michel Vounatsos Contributed to the achievement of record revenues of $13,453 million and $26.20Non-GAAP diluted EPS for the year ended December 31, 2018, versus targets of $12,780 million and $24.74, respectively. Excelled in leading the Company in achieving its financial and business development goals. Added substantial value to our business development activities and the diversification of our pipeline. Contributed significantly to the demonstrated resilience in our MS business, the continued successful launch of SPINRAZA worldwide and the significant progress made in our biosimilars business. Drove our ongoing improvements in our core processes to improve operating efficiencies, capital allocation and asset optimization while adhering to our core values. Jeffrey D. Capello Contributed to the achievement of record revenues of $13,453 million and $26.20Non-GAAP diluted EPS for the year ended December 31, 2018, versus targets of $12,780 million and $24.74, respectively. Significantly improved our Finance organization structure and key processes, including improved financial forecasting and planning and tax and treasury planning. Added substantial value to our business development activities and the diversification of our pipeline. Contributed to the return of approximately $4.4 billion to stockholders in 2018 through share repurchases under our 2018 Share Repurchase Program and 2016 Share Repurchase Program. Contributed to excellent interactions with investors leading to transparent and trusted dialogue. Contributed to improvements in our core processes to improve operating efficiencies, capital allocation and asset optimization while adhering to our core values. Supported our Board of Directors, the CEO and executive team. Michael Ehlers Exceeded portfolio value and clinical development goals. Significantly progressed and developed our pipeline. Significantly improved our Research and Development organization structure, key processes and productivity. Added new capabilities and talent to our Research and Development organization. Excelled in leadership of our Research and Development organization. Added substantial value to our business development activities. Contributed to excellent interactions with investors leading to transparent and trusted dialogue. Susan H. Alexander Supported our Board of Directors, the CEO and executive team and SEC disclosure requirements. Strengthened the intellectual property rights of our key assets, including our intellectual property related to TECFIDERA. Excelled in leadership of our Legal and Compliance teams. Contributed significantly on strategy and the resolution of general business issues affecting the Company, including our expansion into Asia Pacific and Latin America. Supported the effective transition of the corporate services functions, including IT, to Mr. Capello. Paul F. McKenzie Excelled in management of our large and complex manufacturing organization. Maintained excellence in manufacturing plant quality. | | | | | | 45 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
Excelled in leadership of our Pharmaceutical Operations and Technology organization. Contributed significantly on strategy and the resolution of general business issues affecting the Company. Contributed to the significant progress in our biosimilars business. Exhibited outstanding leadership, fostering a culture of continuous improvement and cost-consciousness. In addition, our C&MD Committee reviews on a qualitative basis each named executive officer’s other contributions to the Company and our business, leadership competencies and relative performance among our named executive officers. 2018 Annual Bonus Plan Awards Our C&MD Committee determined that the final bonus awards under our 2018 annual bonus plan were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | Name | | Year-end Salary (A) x | | | Target Bonus% (B) x | | | Company Multiplier (C) x | | | Individual Multiplier (D) = | | | Bonus Award (E) | | | | | | | | | | | | | | M. Vounatsos | | $ | 1,300,000 | | | | 140 | % | | | 131 | % | | | 140 | % | | $ | 3,337,880 | | | | | | | | | | | | | | J. Capello | | $ | 750,000 | | | | 70 | % | | | 131 | % | | | 115 | % | | $ | 790,913 | | | | | | | | | | | | | | M. Ehlers | | $ | 834,094 | | | | 70 | % | | | 131 | % | | | 120 | % | | $ | 917,837 | | | | | | | | | | | | | | S. Alexander | | $ | 749,177 | | | | 70 | % | | | 131 | % | | | 125 | % | | $ | 858,744 | | | | | | | | | | | | | | P. McKenzie | | $ | 633,938 | | | | 70 | % | | | 131 | % | | | 135 | % | | $ | 784,784 | | | | | | | |
Long-Term Incentives | | | | | | | | | | | Terms | | Performance Stock Units (PSUs) | | Market Share Units (MSUs) | | | | Proportion of Annual Target Value | | 50% | | 50% | | | | | Settlement | | 60% stock settled | | 40% cash settled | | 100% stock settled | | | | | | Performance Period(s) | | 3 years (2018-2020) | | 1 year (each of 2018, 2019, 2020) | | 1 year, 2 years, 3 years (from grant date) | | | | | Metrics and Weighting | | AdjustedNon-GAAP diluted EPS: 30% Pipeline Milestone Performance: 30% | | Adjusted Free Cash Flow: 28% Revenues: 12% | | Stock Price: 100% | | | | | Threshold / Maximum Payout (% of Target Award) | | 50% / 200% | | 50% / 200% | | 50% / 200% | | | | | | Vesting | | 3-year Cliff Vesting | | 3-year Cliff Vesting | | Annual Ratable Vesting over 3 years (1/3 per year) | | | | |
All annual LTI awards granted to our executives are performance-based and designed to reward long-term Company performance. Our executive annual LTI program for 2018 consisted of PSUs and MSUs, with the annual LTI total target grant value of awards being split evenly between PSUs and MSUs. The PSUs we awarded to executive officers are performance-based RSUs that are settled, as applicable, in cash and shares of our common stock. The MSUs we awarded to executive officers are performance-based RSUs that are settled in shares of our common stock. The performance conditions applicable to these PSUs and MSUs are described in further detail below. Our annual LTI target grant values are differentiated based on an executive’s individual performance, potential future contributions and market competitiveness, as well as other factors. In determining the annual LTI target grant value, our C&MD Committee reviews LTI awards of our peer group and also reviews the total compensation of our executive officers against our peer group. In general, we have a heavier weighting in executive compensation mix towards LTI awards. On average, annual LTI target grant values for our NEOs position their total compensation at or around the median values of our peer group in cases where there are comparable positions at the peer companies. | | | | | | 46 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
We have an established annual LTI grant practice where LTI grants are made following the completion of our internal performance reviews of our executive officers as well as our external market review of equity practices of our peer group, including the data from the Willis Towers Watson survey described above. Since 2004 we have made our annual LTI grants in February of each year following our annual earnings release. We generally grant time-based RSUs in lieu of PSUs at the time an executive is hired if employment commences after June 30th. These grants are generally granted on the first trading day of the month following the date of hire. From time to time, we also grant time-based RSUs to recognize extraordinary contributions to the Company or for transition or retention purposes. In 2018 the annual LTI target grant values for our NEOs were as follows: | | | | | | | | | Name | | Annual LTI
Target Grant Value | | | | | | | | | M. Vounatsos | | $11,500,000 | | | | | | | | | J. Capello(1) | | n/a | | | | | | | | | M. Ehlers(2) | | $ 3,750,000 | | | | | | | | | S. Alexander(2) | | $ 3,200,000 | | | | | | | | | P. McKenzie(2) | | $ 3,000,000 | | | | | |
Notes to the 2018 Annual LTI Awards Table | (1) | In lieu of a 2018 annual LTI award, Mr. Capello received a new hire grant in January 2018, which consisted of PSUs and MSUs with an aggregate grant date target value of $3.0 million. The initial determination of these awards took into account the Company’s peer group data. |
| (2) | In addition to the annual LTI award, Dr. Ehlers, Ms. Alexander and Dr. McKenzie each received aone-time transition award of RSUs, as described in further detail below. |
The actual value that will be realized from PSU awards depends on the degree of achievement of performance goals, with 60% of the PSUs (based on the grant date target value) settled in shares of our common stock based upon achievement of cumulative three-year financial and pipeline metrics and the remaining 40% of the PSUs settled in cash based upon the achievement of two annual financial metrics that are determined at the beginning of each relevant year. The actual value that will be realized from MSU awards depends on our30-day average common stock price growth between the grant date and each of the dates such awards vest. Our common stock price is influenced by the Company’s performance as well as external market factors. 2018 PSUs PSUs comprised 50% of our executives’ target LTI for 2018. PSUs are performance-based RSUs that have three-year cliff vesting in furtherance of the Company’s long-termpay-for-performance philosophy and to encourage employee retention. PSUs align executive compensation to Company goals through performance against a combination of financial and pipeline milestone performance metrics. The actual value (if any) of PSUs will not be realized by the NEOs until the three-year period ends and then only if the applicable performance goals are achieved. For our 2018 PSU awards, 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock based on achievement of financial and pipeline performance goals over a three-year performance period (the 2018 Stock-Settled PSUs). The remaining 40% of the PSUs will be settled in cash based on the achievement of three sets ofone-year financial goals (the 2018 Cash-Settled PSUs) and continued employment through the vesting date. Our 2018 PSU awards are scheduled to vest in February 2021. For our 2018 PSU awards, the number of PSUs earned at the end of the three-year performance period will be determined as follows: 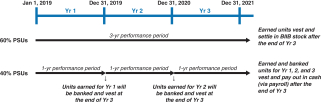
In designing our 2018 PSU LTI program, our C&MD Committee acknowledged the need to balance driving long-term performance and investing for the future with achieving key milestones along the way. Cash payments are primarily aligned with and reward more recent performance, while equity awards encourage our executives to continue to deliver results over a longer period of time and also serve as a retention tool. Accordingly, our C&MD Committee determined that moving compensation for our executive officers further away from cash and towards equity awards with longer-term goals would further align their interests with those of Biogen’s stockholders in creating long-term stockholder value.
| | | | 5 | | Executive Compensation Matters (continued) |
2018 PSU Awards Table Set forth below is a summary of the performance metrics and weightings that our C&MD Committee established for our 2018 PSU awards and the degree to which we achieved the performance goals for the 2018 tranche of the 2018 Cash-Settled PSUs. Based on the results outlined in the table below, the multiplier for the 2018 tranche of the 2018 Cash-Settled PSUs was 192%. | | | | | | | | | | | | | | | | | | | | | | | | Percentage of
PSU Award | | Percentage of PSU Target
Value / Total
LTI Target
Value | | Performance Metrics | | Performance
Metrics
Weight | | | Performance
Period | | | Target Performance | | Actual Performance | Stock- Settled: 60% | | 60% / 30% | | Adjusted Non-GAAP diluted EPS Pipeline Milestone Performance | |
| 30%
30% |
| |
| 2018-2020
2018-2020 |
| | Specific goals are not disclosed for competitive reasons | Cash- Settled: 40% | | 40% / 20% | | Adjusted Free Cash Flows Revenues | | | 28% 12% | | |
| 2018
2019 2020 2018 2019 2020 |
| | $ 2.9B Target set at beginning of 2019 Target set at beginning of 2020 $ 12.8B Target set at beginning of 2019 Target set at beginning of 2020 | | $ 4.0B(1)
TBDTBD $ 13.4B(2)
TBD TBD |
Notes to the 2018 PSU Awards Table | (1) | This financial measure was based on ourNon-GAAP free cash flows, as adjusted to add back $256 million to reflect the cash impact of additional research and development expense recognized in 2018 resulting from the 2018 Ionis Agreement, $16 million to neutralize the unfavorable cash impact of the worldwide withdrawal of ZINBRYTA and $33 million related to higher than originally contemplated stock repurchases in 2018, partially offset by the subtraction of $235 million to reflect tax payments made in connection with tax reform, as these charges were not originally contemplated at the time these performance goals were determined. |
| (2) | This financial measure was based on our publicly reported revenues of $13.5 billion, as adjusted to neutralize the effects of foreign exchange rate fluctuations. |
The 2018 Stock-Settled PSUs metrics were approved by our C&MD Committee with equal weighting assigned to each metric. The two metrics selected were the achievement of a cumulative three-year adjustedNon-GAAP diluted EPS and pipeline milestone performance, in each case, for the three-year period of 2018 through 2020. AdjustedNon-GAAP diluted EPS measured at the end of three-year performance period was selected to reinforce the importance of achieving long-term financial and operational performance. Our C&MD Committee believes that adjustedNon-GAAP diluted EPS is a transparent, operations-based measure. Pipeline milestone performance over the three-year period of 2018 through 2020 was selected to drive our long-term strategic direction and stockholder value creation through our pipeline progress. The 2018 Cash-Settled PSUs financial metrics are adjusted free cash flows and revenues. At the beginning of each year during the performance period for our 2018 PSU awards, our C&MD Committee will approve the targets for each of these financial metrics for such year. Our C&MD Committee decided that because of the nature of our business, in which operating metrics can potentially be impacted positively or negatively by events outside of the control of executives, the design of the PSU program would be based, in part, on the use of threeone-year financial goals. Our C&MD Committee views free cash flow as a critical measure to align the interests of management with those of our stockholders as it reflects the net cash flows available to the Company to pursue opportunities that enhance stockholder value. As such, a cash flow performance goal encourages management to optimize capital expenditures, invest prudently in high return projects and optimize working capital. We selected revenues as a performance measure to reinforce the importance of achieving and exceeding our revenue goal and to provide further incentive to achieve such goal. | | | | | | 48 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
In order to further motivate our executives to drive the organization toward the achievement of these goals, we provide for a maximum payout of 200% for our 2018 PSU awards. Participants may ultimately earn between 0% and 200% of the target number of PSUs granted based on the degree of actual performance goal achievement, generally subject to continued service with the Company. 2018 MSUs MSUs comprised 50% of our executives’ target LTI for 2018. MSUs are performance-based RSUs that are earned based on our common stock price performance from the date of grant to each of the three annual vesting dates. On each vesting date, the performance multiplier is derived based on the stock price growth measured from the grant date to such vesting date using the average closing stock price for the 30 calendar days following and including the grant date and 30 calendar days prior to and including such vesting date for MSUs granted in 2018. Participants may ultimately earn between 0% and 200% of the target number of MSUs awarded based on actual stock performance. The maximum payout percentage of MSUs granted in 2018 is consistent with those granted in 2017 (200%). Once the performance multiplier is determined, it is applied to the target number of MSUs granted to each executive and can increase or decrease the overall number of MSUs earned based on stock price performance. MSU Illustration 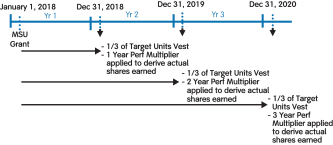
The three-year service vesting period ties executive compensation directly to our common stock price performance, as both the MSUs earned and the value actually received in respect of MSUs are dependent on the performance of our common stock over the vesting period. On each vesting date, the earned MSUs are settled in shares of our common stock. The following table shows the vesting date, performance period and performance multiplier applied for MSUs vesting in 2018 and 2019: | | | | | | | | | | | | | | | | Grant Date | | Vesting Date | | Performance Period | | | Performance Multiplier | | | | | | 2/2018 | | 2/2019 | | | 1 year | | | | 114% | | | | | | 2/2017 | | 2/2019 | | | 2 years | | | | 124% | | | 2/2018 | | | 1 year | | | | 126% | | | | | | 2/2016 | | 2/2019 | | | 3 years | | | | 134% | | | 2/2018 | | | 2 years | | | | 132% | |
2018One-Time Transition Awards As part of our 2018 LTI program change and transition plan, our C&MD Committee decided to grantone-time transition awards in the form of time-based RSUs in February 2018 to certain executive officers, excluding Messrs. Vounatsos and Capello, which vest over atwo-year period, with 33% vesting on the first anniversary of the grant date and 67% vesting on the second anniversary of the grant date. These awards were intended to help mitigate the impact on executives’ compensation and cash flow disruption due to the program changes, including the change to the three-year cliff vesting schedule applied to the PSU awards discussed above compared to the annual installment vesting over three years that applied to the CSPUs that we previously granted. In 2018 theone-time transition awards of RSUs for our NEOs were as follows: | | | | | | Name | | Grant Date Value | | | | | M. Vounatsos | | n/a | | | | | J. Capello | | n/a | | | | | M. Ehlers | | $ 1,500,000 | | | | | S. Alexander | | $ 1,280,000 | | | | | P. McKenzie | | $ 1,200,000 | | |
Retirement Plans We maintain a Supplemental Savings Plan (SSP), which is anon-qualified deferred compensation plan covering our executive officers and other eligible employees in the U.S. We offer the SSP as part of the retirement savings component of our benefits program. We designed the SSP to be competitive with thenon-qualified deferred compensation plans offered by companies in our peer group at that time. Details of the SSP are discussed under the heading “2018Non-Qualified Deferred Compensation” below. | | | | | | 49 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
Other Benefits In addition to eligibility for the benefit programs generally provided to all employees, such as our employee stock purchase plan, 401(k) plan and medical, dental, vision, life and disability insurance, we provide certain supplemental benefits to our executives. These benefits include: Life Insurance All of our U.S. executives, including our NEOs, receive Company-paid term life insurance equal to three times their annual base salary, up to a maximum benefit amount. In 2018 the maximum benefit amount for the CEO was $1.5 million and was $2.25 million for the other NEOs. Employees who are not executives receive Company-paid term life insurance equal to two times their annual base salary. The additional value of Company-provided life insurance for our executive officers reflects competitive practices and is consistent with our philosophy to provide appropriate levels of financial security for our employees based on their positions within the Company. The cost of Company-paid life insurance in excess of a $50,000 insurance level is taxable income to U.S. employees and is not grossed up by the Company. Executive Physicals, Tax Preparation, Financial and Estate Planning Our executive officers, other than our CEO, are eligible for reimbursement of expenses incurred for tax preparation and financial and estate planning services, as well as the purchase of tax preparation and financial planning software, subject to annual expense limits of $7,500 for Executive Vice Presidents. Such reimbursements are taxable income to our executives and are not grossed up. All of our executive officers, including our CEO, are eligible for reimbursement for the cost of their executive physicals, subject to the annual expense limits noted above of $7,500 for our Executive Vice Presidents and CEO. This benefit provides our executives with additional flexibility to proactively manage their health and wellness. Relocation Expenses Under our Executive Relocation Policy, we will, in certain circumstances, provide relocation benefits when employees first join us. Post-Termination Compensation and Benefits We provide severance benefits to all of our executive officers if they are terminated without cause or in certain other circumstances. The terms of these arrangements and the amounts payable under them are described below for each NEO under the heading “Potential Payments Upon Termination or Change in Control.” We provide these benefits because we believe that severance protection is necessary to help our executives maintain their focus on the best interests of the Company when providing advice to the Company and when making strategic decisions about a potential corporate transaction or change in control, and further encourages effective leadership in the closing and integration of significant transactions affecting the Company. Stock Ownership Guidelines We maintain stock ownership guidelines for our executive officers to strengthen and reinforce the link our compensation programs create between our executives and our stockholders. A summary of our stock ownership guidelines is set forth below. | | | | | | Level | | Number of Shares Equal in Value to: | | | CEO | | 6x base salary | | | | | Executive Vice Presidents | | 3x base salary | | |
Executive officers have five years from their initial appointment to meet the requirement. In the event the requirement is not met within that time, 100% of vested shares received in respect of LTI awards are required to be held until the requirement is satisfied. Only stock owned outright or otherwise vested or earned performance-based shares is credited toward the stock ownership requirement. Shares underlying unvested or unearned performance-based shares are not included in the calculation. All of our executive officers currently meet the stock ownership requirement or are still within the five-year period to meet such requirement. Recoupment of Compensation We may recover compensation from our employees, including our executive officers, who engage in detrimental or competitive activity. Detrimental activity includes any action or failure to act that constitutes financial malfeasance that is materially injurious to the Company, violates our Code of Conduct (Values in Action), results in a restatement of our earnings or financial results or results in a violation or breach of law or contract. Competitive activity includes any action or failure to act that violatesnon-disclosure,non-competition and/ornon-solicitation agreements. Our 2008 Performance-Based Management Incentive Plan | | | | | | 50 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
allows for the forfeiture and/or repayment of cash-based awards and our 2008 Omnibus Equity Plan and our 2017 Omnibus Equity Plan each allow for the cancellation of LTI awards in these circumstances as well as the forfeiture of stock or cash acquired upon vesting or sale of LTI awards. In addition, cashsign-on bonuses paid to our NEOs may be subject to repayment if the NEO voluntarily resigns from the Company or if his or her employment is terminated by the Company in certain circumstances. Insider Trading, Hedging and Pledging Policy Prohibitions We maintain a Global Insider Trading and Information Policy that prohibits our employees and directors from, among other things, engaging in hedging or derivative transactions with respect to the Company’s equity securities, purchasing Company stock on margin, pledging Company securities as collateral for a loan or engaging in short sales of the Company’s securities. Tax-Deductibility of Compensation Section 162(m) of the Internal Revenue Code generally limits the amount a company may deduct for compensation in excess of $1 million paid to certain “covered employees,” subject to certain transition relief applicable to certain arrangements in place as of November 2, 2017, and not materially modified after such date. Our C&MD Committee regularly reviews the provisions of our plans and programs, works with its independent compensation consultant and reviews and considers, among other things, the tax deductibility of compensation payments. Our C&MD Committee, however, believes that compensation programs that attract, retain and reward executive talent and achievement are necessary for our success and, therefore, are in the best interests of the Company and our stockholders and that, in establishing the cash and equity incentive compensation programs for the Company’s executive officers, the potential deductibility of the compensation payable under such programs should only be one of a number of relevant factors taken into consideration. Consequently, our C&MD Committee may pay or provide, and has paid or provided, compensation that is not tax deductible in whole or in part. Compensation Committee Report The Compensation and Management Development Committee furnishes the following report: The Compensation and Management Development Committee has reviewed and discussed the Compensation Discussion and Analysis with Biogen management. Based on this review and discussion, the Compensation and Management Development Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Proxy Statement. Submitted by, Robert W. Pangia (Chair) Richard C. Mulligan Eric K. Rowinsky Lynn Schenk | | | | | | 51 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
Summary Compensation Table The following table shows the compensation paid to or earned by our NEOs during the years ended December 31, 2018, December 31, 2017, and December 31, 2016, for the year(s) in which they were a named executive officer. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Name and Principal Position (a) | | Year (b) | | Salary (c) | | Bonus(1) (d) | | Stock Awards(2) (e) | | Non-Equity Incentive Plan Compensation(3) (f) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings(4) (g) | | All Other Compensation(5) (h) | | Total (i) | Michel Vounatsos(6) | | | | 2018 | | | | $ | 1,276,923 | | | | | — | | | | $ | 11,064,897 | | | | | $3,337,880 | | | | | $ 80,663 | | | | | $408,283 | | | | $ | 16,168,646 | | Chief Executive Officer | | | | 2017 | | | | $ | 1,087,885 | | | | | — | | | | $ | 9,868,540 | | | | | $2,502,500 | | | | | $ 18,881 | | | | | $186,567 | | | | $ | 13,664,373 | | | | | | 2016 | | | | $ | 519,231 | | | | $ | 1,500,000 | | | | $ | 3,151,199 | | | | | $ 447,799 | | | | | $ 1,598 | | | | | $181,568 | | | | $ | 5,801,395 | | Jeffrey D. Capello(7) | | | | 2018 | | | | $ | 750,000 | | | | | — | | | | $ | 2,889,224 | | | | | $ 790,913 | | | | | — | | | | | $ 46,582 | | | | $ | 4,476,719 | | Executive Vice President | | | | 2017 | | | | $ | 28,846 | | | | $ | 520,000 | | | | | — | | | | | — | | | | | — | | | | | $ 132 | | | | $ | 548,978 | | | and Chief Financial Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Michael D. Ehlers | | | | 2018 | | | | $ | 829,511 | | | | | — | | | | $ | 5,107,535 | | | | | $ 917,837 | | | | | $ 5,258 | | | | | $186,510 | | | | $ | 7,046,651 | | Executive Vice President, | | | | 2017 | | | | $ | 792,139 | | | | | — | | | | $ | 3,157,338 | | | | | $1,012,034 | | | | | $ 1,799 | | | | | $101,671 | | | | $ | 5,064,981 | | Research & Development | | | | 2016 | | | | $ | 491,827 | | | | $ | 1,170,177 | | | | $ | 3,410,650 | | | | | $ 425,062 | | | | | $ 155 | | | | | $ 14,665 | | | | $ | 5,512,536 | | Susan H. Alexander | | | | 2018 | | | | $ | 746,254 | | | | | — | | | | $ | 4,359,590 | | | | | $ 858,744 | | | | | $188,056 | | | | | $201,140 | | | | $ | 6,353,784 | | Executive Vice President, | | | | 2017 | | | | $ | 720,630 | | | | | — | | | | $ | 3,157,338 | | | | | $ 856,305 | | | | | $148,961 | | | | | $178,008 | | | | $ | 5,061,242 | | Chief Legal Officer | | | | 2016 | | | | $ | 693,663 | | | | | — | | | | $ | 2,337,051 | | | | | $ 589,514 | | | | | $133,726 | | | | | $171,114 | | | | $ | 3,925,068 | | and Secretary | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Paul F. McKenzie | | | | 2018 | | | | $ | 630,455 | | | | | — | | | | $ | 4,083,884 | | | | | $ 784,784 | | | | | $ 12,367 | | | | | $154,406 | | | | $ | 5,665,896 | | Executive Vice President, | | | | 2017 | | | | $ | 600,433 | | | | | — | | | | $ | 3,514,451 | | | | | $ 796,648 | | | | | $ 2,471 | | | | | $101,254 | | | | $ | 5,015,257 | | Pharmaceutical Operations & Technology | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Notes to the Summary Compensation Table | (1) | The amounts in column (d) reflectsign-on bonuses paid to Messrs. Vounatsos and Capello and Dr. Ehlers at the time of hire. All other cash bonuses, which were based on achievement of performance goals under our annual bonus plan, are disclosed in column (f). |
| (2) | The amounts in column (e) reflect the grant date fair value computed in accordance with ASC 718 for RSUs, MSUs, PSUs and CSPUs granted during 2018, 2017 and 2016, as applicable, excluding the effect of estimated forfeitures. The cash portion of PSUs are included in the year when goals are set and fair value is determinable. The 2018 amounts includeone-third of the 2018 Cash-Settled PSUs, which is the tranche of the award for which goals had been set in 2018 relating to the 2018 performance period. The 2018 amounts also includeone-time transition awards of RSUs granted to each of the NEOs, except Messrs. Vounatsos and Capello, as described under “2018One-Time Transition Awards” above. The 2017 amounts for Dr. McKenzie represent grants of MSUs, CSPUs and RSUs, as described in “Executive Compensation Matters—Compensation Discussion and Analysis—2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Arrangement with Dr. McKenzie” in our 2018 proxy statement. The 2016 amounts for Dr. Ehlers represent grants of MSUs, CSPUs and RSUs as described in “Executive Compensation Matters—Compensation Discussion and Analysis—2016 and 2017 Hiring- and Transition-Related Compensation Decisions—Arrangements with Mr. Vounatsos and Dr. Ehlers” in our 2017 proxy statement. The awards granted before February 1, 2017, were subsequently adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts reported in this column were not impacted by such anti-dilution adjustments. The grant date fair value for MSU awards are estimated as of the date of grant using a lattice model with a Monte Carlo simulation, based on the probable outcome of applicable performance conditions. The grant date fair value for PSU, CSPU and RSU awards was determined by multiplying the number of shares subject to the award (assuming target performance for PSUs and CSPUs) by the closing price of the Company’s common stock on the grant date. The assumptions used to calculate the grant date fair value of stock awards are included in footnote 16 of our 2018 Annual Report on Form10-K. The table below shows the target and maximum payouts possible for the 2018, 2017 and 2016 MSU, PSU and CSPU awards based on the value at the date of grant and the target and maximum payout levels. |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 2018 | | | 2017 | | | 2016 | | | | | | | | | | Executive Officer | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | | Mr. Vounatsos | | | $11,064,897 | | | | $22,129,794 | | | | $9,868,540 | | | | $19,737,080 | | | | $3,151,199 | | | | $6,302,398 | | Mr. Capello | | | $ 2,889,224 | | | | $ 5,778,448 | | | | — | | | | — | | | | — | | | | — | | Dr. Ehlers | | | $ 3,608,292 | | | | $ 7,216,584 | | | | $3,157,338 | | | | $ 6,314,676 | | | | $2,540,438 | | | | $5,080,876 | | Ms. Alexander | | | $ 3,078,822 | | | | $ 6,157,644 | | | | $3,157,338 | | | | $ 6,314,676 | | | | $2,337,051 | | | | $4,674,102 | | Dr. McKenzie | | | $ 2,883,856 | | | | $ 5,767,712 | | | | $2,713,851 | | | | $ 5,427,702 | | | | — | | | | — | |
| | | | | | 52 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
| (3) | The amounts in column (f) reflect actual bonuses paid under our annual bonus plan for the applicable year. |
| (4) | The amounts in column (g) reflect earnings in the SSP that are in excess of 120% of the applicable federal long-term rate. The federal long-term rates applied in this calculation are 3.06%, 3.26% and 3.14% for 2018, 2017 and 2016, respectively. The SSP is described under the heading “2018Non-Qualified Deferred Compensation” below. |
| (5) | The amounts in column (h) for 2018 reflect the following: |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Executive Officer | | Company Matching Contribution to 401(k) Plan Account | | Company Contribution to SSP Account | | Personal Health and Financial Planning(8) | | Value of Company- Paid Life Insurance Premiums | | Relocation(9) | Mr. Vounatsos | | | $ | 16,500 | | | | $ | 387,134 | | | | $ | 3,594 | | | | $ | 1,055 | | | | | — | | Mr. Capello | | | $ | 16,500 | | | | $ | 28,500 | | | | | — | | | | $ | 1,582 | | | | | — | | Dr. Ehlers | | | $ | 16,500 | | | | $ | 168,428 | | | | | — | | | | $ | 1,582 | | | | | — | | Ms. Alexander | | | $ | 16,500 | | | | $ | 176,553 | | | | $ | 6,560 | | | | $ | 1,527 | | | | | — | | Dr. McKenzie | | | $ | 16,500 | | | | $ | 118,612 | | | | $ | 5,179 | | | | $ | 1,274 | | | | $ | 12,841 | |
| (6) | Mr. Vounatsos joined Biogen as our Executive Vice President, Chief Commercial Officer effective April 18, 2016. Mr. Vounatsos was appointed our Chief Executive Officer and a member of our Board of Directors effective January 6, 2017. His base salary for 2017 was $1,100,000, of which he received a pro rata share from January 6, 2017 to December 31, 2017. From January 1, 2017 to January 5, 2017, he was paid at his prior rate of base salary ($750,000). |
| (7) | Mr. Capello was appointed as our Executive Vice President and Chief Financial Officer effective December 11, 2017. His base salary for 2017 was $750,000, of which he received a pro rata share from December 11, 2017 to December 31, 2017. Mr. Capello was not eligible to participate in our 2017 annual bonus plan and was not granted any stock awards in 2017. |
| (8) | Represents reimbursements of expenses relating to tax, financial and estate planning and executive physicals as described under the heading “Executive Physicals, Tax Preparation, Financial and Estate Planning” above. |
| (9) | The amount for Dr. McKenzie reflects relocation benefits under the Company’s Executive Relocation Policy and includes a taxgross-up of $1,518. |
| | | | | | 53 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
2018 Grants of Plan-Based Awards The following table shows additional information regarding all grants of plan-based awards made to our NEOs for the year ended December 31, 2018. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Estimated Future Payouts Under Non-Equity Incentive Plan
Awards(1) | | | | Estimated Future Payouts
Under Equity Incentive Plan
Awards(#)(1) | | All Other Stock Awards: Number of
Shares or Units
(#) (i) | | Grant Date Fair Value of Stock Awards(2) (j) | Name (a) | | Grant Date (b) | | Notes | | Threshold (c) | | Target (d) | | Maximum (e) | | | | Threshold (f) | | Target (g) | | Maximum (h) | Michel Vounatsos | | | | 02/12/2018 | | | (3) | | | | — | | | | | — | | | | | — | | | | | | | | | | 9,080 | | | | | 18,160 | | | | | 36,320 | | | | | — | | | | $ | 6,856,251 | | | | | | 02/12/2018 | | | (4) | | | | — | | | | | — | | | | | — | | | | | | | | | | 6,646 | | | | | 13,292 | | | | | 26,584 | | | | | — | | | | $ | 4,208,646 | | | | | | 02/12/2018 | | | (5) | | | $ | 455,000 | | | | $ | 1,820,000 | | | | $ | 4,095,000 | | | | | | | | | | — | | | | | — | | | | | — | | | | | — | | | | | — | | Jeffrey D. Capello | | | | 01/02/2018 | | | (3) | | | | — | | | | | — | | | | | — | | | | | | | | | | 2,245 | | | | | 4,490 | | | | | 8,980 | | | | | — | | | | $ | 1,789,136 | | | | | | 01/02/2018 | | | (4) | | | | — | | | | | — | | | | | — | | | | | | | | | | 1,646 | | | | | 3,292 | | | | | 6,584 | | | | | — | | | | $ | 1,100,088 | | | | | | | 02/12/2018 | | | (5) | | | $ | 131,250 | | | | $ | 525,000 | | | | $ | 1,181,250 | | | | | | | | | | — | | | | | — | | | | | — | | | | | — | | | | | — | | Michael D. Ehlers | | | | 02/12/2018 | | | (3) | | | | — | | | | | — | | | | | — | | | | | | | | | | 2,960 | | | | | 5,920 | | | | | 11,840 | | | | | — | | | | $ | 2,235,068 | | | | | | 02/12/2018 | | | (4) | | | | — | | | | | — | | | | | — | | | | | | | | | | 2,169 | | | | | 4,337 | | | | | 8,674 | | | | | — | | | | $ | 1,373,224 | | | | | | 02/12/2018 | | | (5) | | | $ | 145,966 | | | | $ | 583,866 | | | | $ | 1,313,698 | | | | | | | | | | — | | | | | — | | | | | — | | | | | — | | | | | — | | | | | | | 02/12/2018 | | | (6) | | | | — | | | | | — | | | | | — | | | | | | | | | | — | | | | | — | | | | | — | | | | | 4,735 | | | | $ | 1,499,243 | | Susan H. Alexander | | | | 02/12/2018 | | | (3) | | | | — | | | | | — | | | | | — | | | | | | | | | | 2,528 | | | | | 5,055 | | | | | 10,110 | | | | | — | | | | $ | 1,908,558 | | | | | | 02/12/2018 | | | (4) | | | | — | | | | | — | | | | | — | | | | | | | | | | 1,848 | | | | | 3,696 | | | | | 7,392 | | | | | — | | | | $ | 1,170,264 | | | | | | 02/12/2018 | | | (5) | | | $ | 131,106 | | | | $ | 524,424 | | | | $ | 1,179,954 | | | | | | | | | | — | | | | | — | | | | | — | | | | | — | | | | | — | | | | | | | 02/12/2018 | | | (6) | | | | — | | | | | — | | | | | — | | | | | | | | | | — | | | | | — | | | | | — | | | | | 4,045 | | | | $ | 1,280,768 | | Paul F. McKenzie | | | | 02/12/2018 | | | (3) | | | | — | | | | | — | | | | | — | | | | | | | | | | 2,368 | | | | | 4,735 | | | | | 9,470 | | | | | — | | | | $ | 1,787,683 | | | | | | 02/12/2018 | | | (4) | | | | — | | | | | — | | | | | — | | | | | | | | | | 1,731 | | | | | 3,462 | | | | | 6,924 | | | | | — | | | | $ | 1,096,173 | | | | | | 02/12/2018 | | | (5) | | | $ | 110,939 | | | | $ | 443,757 | | | | $ | 998,452 | | | | | | | | | | — | | | | | — | | | | | — | | | | | — | | | | | — | | | | | | | 02/12/2018 | | | (6) | | | | — | | | | | — | | | | | — | | | | | | | | | | — | | | | | — | | | | | — | | | | | 3,790 | | | | $ | 1,200,028 | |
Notes to the 2018 Grants of Plan-Based Awards Table | (1) | Reflects the potential future payouts of awards granted in 2018 under our 2018 annual bonus plan and our LTI program for each NEO as of the respective grant dates. |
| (2) | Represents the grant date fair value of PSUs, MSUs and RSUs, computed in accordance with ASC 718, excluding the effect of estimated forfeitures. The grant date fair value for MSU awards is estimated as of the date of grant using a lattice model with a Monte Carlo simulation based on the probable outcome of applicable performance conditions. The grant date fair value for both PSU and RSU awards is determined by multiplying the number of shares subject to the award (assuming target performance for PSUs) by the closing price of our common stock on the grant date. For the 2018 Cash-Settled PSUs, the grant date value ofone-third of the award is included, which is the tranche of the award for which goals had been set relating to the 2018 performance period and fair value was determinable in 2018. The remaining tranches of the 2018 Cash-Settled PSUs will be included in compensation tables for 2019 and 2020, the years when goals will be set with respect to such performance periods and fair value is determinable. The assumptions used to calculate the grant date fair value of stock awards are included in footnote 16 of our 2018 Annual Report on Form10-K. The maximum payouts for these awards are included in the footnotes following the Summary Compensation Table above. |
| (3) | These amounts relate to the annual grant of MSUs. MSU grants represent 50% of the annual LTI grant date target value (excludingone-time RSU grants). These are performance-based RSUs tied to the growth in our stock price between the grant date and each of three annual vesting dates. The number of MSUs earned is determined on each vesting date. Columns (f), (g) and (h) represent the number of MSUs that can be earned based on performance at the threshold level of 50%, target level of 100% and the maximum level of 200%, respectively. To the extent earned, the award becomes eligible to vest ratably over three years, generally subject to continued service, as described in further detail under the heading “Long-Term Incentives” above. |
| (4) | These amounts relate to the annual grant of PSUs. PSU grants represent 50% of the annual LTI grant date target value (excludingone-time RSU grants). The PSUs have three-year cliff vesting. The amounts shown include the 2018 Stock-Settled PSUs andone-third of the 2018 Cash-Settled PSUs, which is the tranche of the award for which goals had been set in 2018 relating to the 2018 performance period and fair value was determinable in 2018. The remaining tranches of the 2018 Cash-Settled PSUs will be included in compensation tables for 2019 and 2020, the years when goals will be set with respect to such performance periods and fair value is determinable. Columns (f), (g) and (h) represent the number of 2018 Stock-Settled PSUs and the 2018 tranche of the 2018 Cash-Settled PSUs that can be earned if the Company Multiplier were 50%, 100% and 200%, respectively. For additional information on our PSU awards, please see “Long-Term Incentives—2018 PSUs” above. |
| (5) | These amounts relate to our 2018 annual bonus plan. The amounts shown in column (d) represent the 2018 target payout amount based on the target percentage applied to each NEO’s base salary as of December 31, 2018. For 2018 the bonus targets were 140% of base salary for Mr. Vounatsos and 70% of base salary for all other NEOs. The amounts in column (c), (d) and (e) represent a payment if the Company Multiplier and the Individual Multiplier were each 50%, 100% and 150%, respectively. Actual amounts paid to each NEO |
| | | | | | 54 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
| under our 2018 annual bonus plan are included in the“Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table above. |
| (6) | These amounts relate toone-time transition awards of RSUs granted to each of the NEOs, except Messrs. Vounatsos and Capello, as described under “2018One-Time Transition Awards” above. |
Outstanding Equity Awards at 2018 FiscalYear-End The following table summarizes the equity awards that were outstanding as of December 31, 2018, for each of our NEOs. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Option Awards | | | Stock Awards | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Equity Incentive Plan
Awards | | | | | | | | | | | | | | | Grant
Date (b) | | | Notes | | Number of Securities Underlying Unexercised
Options | | | Option Exercise Price (e) | | | Option Expiration Date (f) | | | Number of Shares or Units of Stock That Have Not Vested (g) | | | Market Value of Shares or Units of Stock That Have Not Vested (h) | | | Number of Unearned Shares or Units That Have Not Vested (i) | | | Market Value of Unearned Shares or Units That Have Not Vested(1) (j) | | | (a) | | Exercisable (c) | | | Unexercisable (d) | | Michel Vounatsos | | | 5/2/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 2,320 | | | $ | 698,134 | | | | — | | | | — | | | | | 5/2/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3,252 | | | $ | 978,592 | | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 14,080 | | | $ | 4,236,954 | | | | — | | | | — | | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 17,002 | | | $ | 5,116,242 | | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 36,320 | | | $ | 10,929,414 | | | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 4,602 | | | $ | 1,384,834 | | | | 15,763 | | | $ | 4,743,402 | | Jeffrey D. Capello | | | 1/2/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,490 | | | $ | 1,351,131 | | | | | | 1/2/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,146 | | | $ | 344,854 | | | | 3,893 | | | $ | 1,171,482 | | Michael D. Ehlers | | | 6/1/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 1,821 | | | $ | 547,975 | | | | — | | | | — | | | | | 6/1/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,502 | | | $ | 752,902 | | | | | 6/1/2016 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 1,036 | | | $ | 311,753 | | | | — | | | | — | | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 4,504 | | | $ | 1,355,344 | | | | — | | | | — | | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,442 | | | $ | 1,637,607 | | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 11,840 | | | $ | 3,562,893 | | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,501 | | | $ | 451,681 | | | | 5,143 | | | $ | 1,547,632 | | | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 4,735 | | | $ | 1,424,856 | | | | — | | | | — | | Susan H. Alexander | | | 2/24/2009 | | | (6) | | | 6,395 | | | | — | | | | $48.52 | | | | 2/23/2019 | | | | — | | | | — | | | | — | | | | — | | | | | 2/22/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 1,806 | | | $ | 543,462 | | | | — | | | | — | | | | | 2/22/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,482 | | | $ | 746,883 | | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 4,504 | | | $ | 1,355,344 | | | | — | | | | — | | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,442 | | | $ | 1,637,607 | | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 10,110 | | | $ | 3,042,301 | | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,279 | | | $ | 384,877 | | | | 4,384 | | | $ | 1,319,233 | | | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 4,045 | | | $ | 1,217,221 | | | | — | | | | — | | Paul F. McKenzie | | | 3/1/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 616 | | | $ | 185,367 | | | | — | | | | — | | | | | 3/1/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 848 | | | $ | 255,180 | | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 3,875 | | | $ | 1,166,065 | | | | — | | | | — | | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,674 | | | $ | 1,406,500 | | | | | 3/1/2017 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 1,820 | | | $ | 547,674 | | | | — | | | | — | | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 9,470 | | | $ | 2,849,712 | | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,194 | | | $ | 359,298 | | | | 4,108 | | | $ | 1,236,179 | | | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 3,790 | | | $ | 1,140,487 | | | | — | | | | — | |
Notes to the Outstanding Equity Awards at 2018 Fiscal Year End Table | (1) | The market value of awards is based on the closing price of our stock on December 31, 2018 ($300.92), as reported by Nasdaq. |
| (2) | CSPUs were granted in 2017 and 2016. The numbers in column (g) reflect the number of CSPUs that were earned based on our financial performance for each of 2017 and 2016, but that had not vested based on our service-based vesting requirement as of December 31, 2018. CSPUs that have been earned based on the satisfaction of the performance conditions vest ratably over three years from the grant date. The cash payout for these awards will be based on our30-day average closing stock price at vesting. Grants made before February 1, 2017, were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts. |
| (3) | MSUs were granted in 2018, 2017 and 2016. These are performance-based RSUs earned based on the growth in our stock price between the dates of grant and vesting. Earned MSUs are eligible to vest ratably over three years. The number and value shown in columns (i) and (j), respectively, reflects maximum performance results for MSUs granted in 2018, 2017 and 2016 based on the estimated performance atyear-end in each case except for Mr. Capello’s January 2, 2018, grant, for which columns (i) and (j) reflect target performance results based on the estimated performance atyear-end. Grants made before February 1, 2017, were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts. |
| | | | | | 55 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
| (4) | PSUs were granted in 2018. The PSUs are subject to three-year cliff vesting. In addition, 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock and performance will be based upon achievement of cumulative three-year financial and pipeline goals. The remaining 40% of the PSUs will be settled in cash and performance will be based upon the achievement of three annual financial goals determined at the beginning of each relevant year. The number and value shown in columns (g) and (h), respectively, reflect the number of 2018 Cash-Settled PSUs that were earned based on our achievement of the annual performance goals for the 2018 tranche, but that will not vest until February 12, 2021. The number and value shown in columns (i) and (j), respectively, reflect the remaining portion of the PSUs granted in 2018 (including the 2019 and 2020 tranches of the 2018 Cash-Settled PSUs) assuming target performance results. For additional information on our PSU awards, please see “Long-Term Incentives—2018 PSUs” above. |
| (5) | RSUs were granted in 2018, 2017 and 2016 for special circumstances. 2018 RSU awards relate toone-time transition awards granted to each of the NEOs, except Messrs. Vounatsos and Capello, as described under “2018One-Time Transition Awards” above. For Dr. Ehlers and Dr. McKenzie, the amounts in this column also reflect 3,104 RSUs granted to Dr. Ehlers on June 1, 2016, in connection with his hiring and 2,730 RSUs granted to Dr. McKenzie on March 1, 2017, under hisone-time award, as described in more detail under the heading “Executive Compensation Matters—Compensation Discussion and Analysis—2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Arrangement with Dr. McKenzie” in our 2018 proxy statement, in each case vesting ratably over three years from the grant date. Grants made before February 1, 2017, were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts. |
| (6) | These stock options were granted with aten-year term. Stock options vest 25% on each of the first four anniversaries of the grant date. These stock options were adjusted pursuant to the anti-dilution provisions of the award in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share and exercise price amounts. |
2018 Option Exercises and Stock Vested Our executive officers must usepre-established trading plans to sell shares of our common stock. Trading plans may only be entered into during an open trading window and when the executive is not in possession of materialnon-public information about the Company, and we require a waiting period following the establishment of a trading plan before any trades may be executed. Our policy is designed to provide safeguards while allowing our executives an opportunity to realize the value intended by the Company in granting equity-based LTI awards. Our NEOs are also subject to the stock ownership guidelines described above under the heading “Stock Ownership Guidelines.” The following table shows information regarding vesting of stock awards for each NEO during the year ended December 31, 2018. None of the NEOs exercised stock options during the year ended December 31, 2018. | | | | | | | | | | | | | | | | | | | | | Stock Awards | | Name | | Number of Shares
Acquired on
Vesting(1) | | | | Value
Realized on
Vesting(2)(3) | Michel Vounatsos | | | | 16,294 | | | | | | | | | $ | 5,013,149 | | Jeffrey D. Capello | | | | — | | | | | | | | | | — | | Michael D. Ehlers | | | | 8,115 | | | | | | | | | $ | 2,471,489 | | Susan H. Alexander | | | | 9,111 | | | | | | | | | $ | 2,844,100 | | Paul F. McKenzie | | | | 5,419 | | | | | | | | | $ | 1,672,605 | |
Notes to the 2018 Option Exercises and Stock Vested Table | (1) | CSPUs were settled in cash for all of our NEOs. The number of actual shares of our common stock acquired on vesting with respect to MSUs and RSUs, as applicable, after shares were withheld to pay the minimum withholding of taxes was as follows: |
| | | | | | | Net Shares Acquired(4) | Mr. Vounatsos | | 4,414 | Mr. Capello | | — | Dr. Ehlers | | 2,545 | Ms. Alexander | | 2,586 | Dr. McKenzie | | 1,830 |
| | | | | | 56 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
| (2) | The value realized for MSUs and RSUs are calculated by multiplying the closing price of a share of our common stock on the vesting date by the total number of shares that vested on such date. The value realized for CSPUs is calculated using the30-day average closing price of the common stock of the Company through the vesting date. |
| (3) | The value realized upon vesting for Dr. McKenzie includes CSPUs with a value of $304,487, the receipt of which was deferred under the SSP, as described below. Terms of thenon-qualified deferred compensation plan are described under the heading “2018Non-Qualified Deferred Compensation” below. |
| (4) | MSUs and RSUs were settled in shares of our common stock. CSPUs were settled in cash. |
2018Non-Qualified Deferred Compensation The SSP covers our executive officers and other eligible employees in the U.S. whose base salary and annual cash incentives for the year exceed a specified Internal Revenue Services limit ($275,000 in 2018) receive a Company-paid restoration match on the portion of their base salary, annual bonus and cash payments in respect of CSPUs and PSUs, as applicable, that exceeds this limit; the restoration match equals 6% of this excess compensation. The restoration match feature is intended to provide the amount of matching employer contributions that the participant would otherwise have been eligible to receive under our 401(k) plan but for the $275,000 limit imposed by Section 401(a)(17) of the Internal Revenue Code. In addition, eligible employees may make voluntary contributions of up to 80% of their base salary and 100% of their annual bonus and cash payments in respect of CSPUs and PSUs, as applicable, to the SSP, and thereby defer income taxes on such amounts until distribution is made from the SSP. The Company does not match participants’ voluntary contributions to the SSP. The SSP provides for immediate vesting of the restoration match consistent with our immediate vesting of the Company match provided under our 401(k) plan. Notional SSP accounts are maintained for each participant. Accounts include employee and employer contributions and reflect the performance of notional investments selected by the employee or a default investment if the employee does not make a selection. These notional investment options include the mutual funds offered under our 401(k) plan as well as a fixed rate option which earns a rate of return determined each year by the Company’s retirement committee. For contributions to the SSP fixed rate option in 2018, this rate of return was set at 5%. Contributions to the fixed rate option continue to earn interest at the rate of return that was in effect during the year of contribution. The excess of the interest rate paid on the fixed rate option above 120% of the applicable federal long-term rate (compounded quarterly) earned by our NEOs during 2018 is shown in the Summary Compensation Table. We fund the SSP liabilities through corporate-owned life insurance (COLI), which we purchase with the written consent of SSP participants, and investments in mutual funds. We believe that the COLI policies and mutual funds will be sufficient to cover plan liabilities through the projected payout date so the plan will not require direct funding by the Company. Upon enrollment in the SSP, a participant must elect when and how distributions will be made from the participant’s account. Distributions can be made upon termination of the participant’s employment, either in a lump sum or up to 15 annual installments, or at a specified future date while the participant is still employed (an“in-service” distribution), either in a lump sum or up to 5 annual installments. Further, upon enrollment, a participant must also elect a distribution method upon death or a change in control of the Company, which can either be a lump sum payment or, if different, the method selected for payment upon termination of employment. The following table shows a summary of all contributions to, earnings on and distributions received from the SSP for each of our NEOs for the year ended December 31, 2018. The account balances as ofyear-end include all contributions and interest amounts earned by our NEOs through the end of 2018 plus the SSP contributions that the Company made in early 2019 based on earnings in the last quarter of 2018. | | | | | | | | | | | | | | | | | | | | | | | | | | Name | | Executive
Contributions
in Last Fiscal
Year(1) | | | Company
Contributions
in Last Fiscal
Year(2) | | | Aggregate
Earnings
in Last
Fiscal
Year(3) | | | Aggregate
Distributions
in Last
Fiscal Year | | Aggregate
Balance at
Last Fiscal Year-End(4) | | Michel Vounatsos | | $ | 3,071,852 | | | $ | 387,134 | | | $ | 205,737 | | | — | | $ | 5,160,044 | | Jeffrey D. Capello | | | — | | | $ | 28,500 | | | $ | 60 | | | — | | $ | 28,560 | | Michael D. Ehlers | | $ | 552,463 | | | $ | 168,428 | | | $ | (80,992 | ) | | — | | $ | 1,270,192 | | Susan H. Alexander | | $ | 826,451 | | | $ | 176,553 | | | $ | 392,167 | | | — | | $ | 7,259,032 | | Paul F. McKenzie | | $ | 860,425 | | | $ | 118,612 | | | $ | 2,629 | | | — | | $ | 1,320,342 | |
| | | | | | 57 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
Notes to the 2018Non-Qualified Deferred Compensation Table | (1) | The amounts in this column are also included, in part, in columns (c), (e) and/or (f) of the Summary Compensation Table and represent deferral of salary, deferral of payments under our 2018 annual bonus plan and deferral of CSPU payments, respectively. |
| (2) | The amounts in this column are also included in column (h) of the Summary Compensation Table for 2018 as Company contributions to the SSP. |
| (3) | Earnings in excess of 120% of the applicable federal long-term rate (compounded quarterly) earned by our NEOs during 2016 is shownare reported in column (g) of the Summary Compensation Table. We fund the SSP liabilities through corporate owned life insurance (COLI) which we purchase with the written consent of SSP participants. We believe that the COLI policies will be sufficient to cover plan liabilities through the projected payout date so the plan will not require direct funding by the Company. Upon enrollment in the SSP, a participant must elect whenTable for 2018 for Mr. Vounatsos ($80,663), Dr. Ehlers ($5,258), Ms. Alexander ($188,056) and how distributions will be made from the participant’s account. Distributions can be made upon termination of the participant’s employment, either in a lump sum or up to 15 annual installments, or at a specified future date while the partic-Dr. McKenzie ($12,367). ipant is still employed (an“in-service” distribution), either in a lump sum or up to five annual installments. Further, upon enrollment, a participant must also elect a distribution method upon death or a change in control of the Company, which can either be a lump sum payment or, if different, the method selected for payment upon termination of employment.
|
| (4) | The following table shows a summary of all contributions to, earnings onlists the compensation deferrals during 2018 and distributions received from2017 by the SSP for each of our NEOs, for the year ended December 31, 2016. The account balances as ofyear-end include all contributions and interest amounts earned by our NEOs through the end of 2016 plus the SSP contributions that the Company made in early 2017 based on earningsreported, where applicable, in the last quarterproxy statement for our 2018 and 2017 Annual Meetings of 2016.Stockholders: | | | | | | | | | | | | | | | | | | | | | | Name | | Executive
Contributions
in Last Fiscal
Year(1) | | | Company
Contributions
in Last Fiscal
Year(2) | | | Aggregate
Earnings
in Last
Fiscal
Year(3) | | | Aggregate
Distributions
in Last
Fiscal Year | | | Aggregate
Balance at
Last Fiscal
Year-End(4) | | George A. Scangos | | $ | 504,375 | | | $ | 446,538 | | | $ | 656,387 | | | | — | | | $ | 11,619,411 | | Michel P. Vounatsos | | $ | 300,000 | | | $ | 18,000 | | | $ | 3,763 | | | | — | | | $ | 321,763 | | Paul J. Clancy | | $ | 0 | | | $ | 176,179 | | | $ | 107,017 | | | | — | | | $ | 1,851,529 | | John G. Cox | | $ | 928,065 | | | $ | 156,382 | | | $ | 553,539 | | | | — | | | $ | 13,995,903 | | Michael D. Ehlers | | $ | 116,250 | | | $ | 13,610 | | | $ | 2,331 | | | | — | | | $ | 132,190 | |
Notes to the 2016Non-Qualified Deferred Compensation Table
(1) | The amounts in this column are also included, in part, in columns (c), (e), and/or (f) of the Summary Compensation Table asnon-qualified deferral of salary,non-qualified deferral of CSPU payments, andnon-qualified deferral of payments under our 2016 annual bonus plan, respectively. |
(2) | The amounts in this column are also included in column (h) of the Summary Compensation Table for 2016 as Company contributions to the SSP. |
(3) | Earnings in excess of 120% of the applicable federal long-term rate are reported in column (g) of the Summary Compensation Table for 2016 for Dr. Scangos ($221,642), Mr. Vounatsos ($1,598), Mr. Clancy ($55,376), Mr. Cox ($247,644), and Dr. Ehlers ($155). |
(4) | The following table lists the compensation deferrals during 2015 and 2014 by the NEOs, as reported, where applicable, in the proxy statement for our 2016 and 2015 Annual Meetings of Stockholders. |
| | | | | | | | | | Amounts Previously
Reported as Deferred | | Name | | 2015 | | | 2014 | George A. Scangos | | $ | 1,368,040 | | | $1,205,644 | John G. Cox | | $ | 3,030,054 | | | $4,313,520 |
| This column also includes Company contributions and compensation earned and deferred in prior years, which was disclosed in our prior proxy statements where applicable, together with earnings on these amounts. |
| | | | | 50 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued) |
| | | | | | | | | | | | | | Amounts Previously
Reported as Deferred | | | | | | Name | | 2017 | | | 2016 | | Mr. Vounatsos | | $ | 1,025,867 | | | $ | 300,000 | | Mr. Capello | | | — | | | | — | | Dr. Ehlers | | $ | 365,160 | | | $ | 116,250 | | Ms. Alexander | | $ | 573,897 | | | $ | 259,816 | | Dr. McKenzie | | $ | 221,128 | | | | — | |
| Potential Payments Upon Termination or ChangeThis column also includes Company contributions and compensation earned and deferred in Control
Executive Severance Policy
Definition of Key Terms Relating to our Executive Severance Plan
Our Executive Severance Policy and benefits refer to certain key terms. These terms are definedprior years, which was disclosed in our 2008prior proxy statements where applicable, together with earnings on these amounts.
|
Potential Payments Upon Termination or Change in Control Executive Severance Policy Definition of Key Terms Relating to our Executive Severance Policy Our executive severance policy and benefits refer to certain key terms. These terms are defined in our 2017 Omnibus Equity Plan. Executive Vice President Arrangements Each of our current NEOs, other than Dr. Scangos, participated inMr. Vounatsos, were covered by our executive severance planspolicy in 20162018 under which they were eligible to receive the following benefits if certain events occurred during 2016:2018: In the event of a termination of employment other than for cause and other than by reason of the executive’s death or disability, the NEO would be entitled to receive a lump sum severance payment equal to a minimum of twelve12 months of such NEO’s then-annualthen base salary and target annual bonus, with an additional two2 months of base salary and target bonus for each full year of service to a maximum benefit of 21 months.months of base salary and target bonus. We refer to the number of months of severance a NEO is entitled to receive as the “severance period”.period.” If, within two years following a corporate transaction or a corporate change in control, the NEO experiences a termination of employment other than for cause orand other than by reason of death or disability or experiences an involuntary employment action, the NEO would be entitled to a lump sum severance payment equal to two times the NEO’s then-annual base salary plus target annual bonus. These payments are in lieu of any payment in the preceding paragraph. | | an involuntary employment action, the NEO would be entitled to a lump sum severance payment equal to two times the NEO’s then-annual base salary plus target annual bonus. These payments are in lieu of any payment in the preceding paragraph. |
The payment of these severance benefits is conditioned upon execution of an irrevocable release in favor of the Company. These The executive severance arrangements dopolicy does not pay severance upon a termination for cause, voluntary resignation, retirement or death or disability. In any case where severance is payable under our executive severance policy, our NEOs would also receive continuation of medical, dental and vision insurance benefits until the earlier of the last date of the severance period or the date the executive becomes eligible to participate in another employer’s medical, dental and vision insurance plans. NEOs would also be provided up to 12 months of executive-level outplacement services at our cost. Annual Bonus Plan Our annual bonus plan provides for a prorated target bonus payment upon a termination of employment due to the death or disability of the participant, and upon a termination of employment arising from an involuntary employment action.participant. As our annual bonus plan provides for payment of a full bonus to any participant remaining employed on the last day of the plan year, annual bonus amounts are not included in the Potential Post-TerminationPost- Termination Payments Table below. In any case where severance is payable under our executive severance plans, our NEOs would also receive con-
tinuation of medical and dental insurance benefits until the earlier of the last date of the severance period or the date the executive becomes eligible to participate in another employer’s medical and dental insurance plans. NEOs are also provided up to twelve months of executive-level outplacement services at our cost.
| | | | | | 58 | |  | |  |
Dr. Scangos’ Arrangements
| | | | 5 | | Executive Compensation Matters (continued) |
On January 6, 2017, Dr. Scangos’ ceased to be our Chief Executive Officer and the Company paid him the severance benefits payable under his employment agreement, described in the CD&A above under the heading “2016 and 2017 Hiring- and Transition-Related Compensation Decisions — Dr. Scangos’ Arrangements.”
Mr. Vounatsos’ Arrangements We entered into an employment agreement with Mr. Vounatsos oneffective January 6, 2017, with an initial term ending on December 31, 2019, at which time the term automatically extends for additional twelve month12-month periods until the agreement is otherwise terminated in accordance with its terms. Under Mr. Vounatsos’ employment agreement, if his employment is terminated by the Company without cause or if he terminates his employment for good reason (referred to in his employment agreement as an involuntary employment action), then he would be entitled to a lump sum payment of cash severance in the amount of one andone-half times his annual base salary and target annual bonus. If, however, such termination of employment occurs within two years following a corporate transaction or a corporate change in control (CIC), then he would be entitled to a lump sum payment of cash severance in the amount of two times his annual base salary and target annual bonus. Mr. Vounatsos would also receive continuation of medical, dental and dentalvision benefits until the earlier of 18 months (24 months if within two2 years of a CIC) following the date his employment terminates or the date upon which he becomes eligible to receive substantially comparable benefits through another employer. In addition, he would be entitled to receive a pro rata portion of his annual cash bonus for the year that such termination occurs based on actual performance or, in the event the termination occurs within two years following a CIC, thehis target annual bonus. Mr. Vounatsos would also be provided executive-level outplacement services for a12-month period following the termination date at our cost. The payment of Mr. Vounatsos’ severance benefits is conditioned upon execution of general release in favor of the Company. During 2017, Mr. Vounatsos has agreed to invest $1.0 million in Biogen common stock.
| | | | | 51 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued) |
Dr. Ehlers’ Additional Arrangements
In connection with the hiring of Dr. Ehlers, we agreed that if a new CEO were appointed within two years from his start date (i.e., prior to May 9, 2018) and as a result of the appointment (1) Dr. Ehlers’ employment is terminated other than for cause or (2) if Dr. Ehlers terminates his employment, following a notice and cure period, because there is a material and substantial change in the Company’s financial spend committed to the R&D organization or a material alteration and diminution in Dr. Ehlers’ authority, duties, or responsibilities, then he would be entitled to receive severance benefits and accelerated vesting of outstanding LTI as if a change in control and involuntary employment action had occurred under our executive severance plan and our 2008 Omnibus Equity Plan.
Mr. Cox’s Additional Arrangements
We entered into an offer letter agreement with Mr. Cox to become the CEO of Bioverativ effective May 19, 2016. The agreement included the following enhanced severance benefits: (i) 24 months of severance instead of 21 months of severance for an involuntary termination, other than “for cause” and (2) the vesting in full of all of his outstanding awards under the Company’s LTI program in the event thespin-off of Bioverativ did not occur and the Executive Vice President, Pharmaceutical Operations and Technology and Global Therapeutic Operations position was no longer available.
Excise Tax Provisions Before June 2009 we maintained an excise taxgross-up policy for all of our executives, including certain of our NEOs. Under this policy, if payments to these executive officers in the event of a corporate transaction or corporate change in control were subject to the excise tax under Internal Revenue Code Section 4999, we would pay the executive officer an additional amount that equals the amount of the excise tax, plus the income and other payroll taxes arising from our payment of the excise tax amount (280G taxgross-up), so that the executive officer realized the full intended benefit. In June 2009 we changed our excise taxgross-up policy so that newly-hired executives are not eligible for any 280G taxgross-up but may elect to have severance payments reduced to an amount that will not be subject to excise tax. Consistent with this policy, Dr. Scangos, Mr. Vounatsos, and Dr. Ehlers are not eligible for a 280G taxgross-up. As Mr. Clancyas Ms. Alexander was already eligible for this benefit prior to June 2009, heshe remains eligible for a 280G taxgross-up. Until his separation from Biogen on January 31, 2017 as a result of the Bioverativspin-off, Mr. Cox had been gross up. No other NEO is eligible for a 280G taxgross-up.this benefit.
Awards Under Equity Plans Under the provisions of our equity plans, if a corporate change in control occurs, all outstanding options and stock awards under our equity plans granted prior to February 12, 2014 will become fully exercisable or vested, as the case may be, and options will remain exercisable until the original option expiration date. As amended in February 2014, awards granted under our 2008 Omnibus Equity Plan on or after February 12, 2014,and our 2017 Omnibus Equity Plan, unless otherwise determined by our CompensationC&MD Committee at the time of grant, will not automatically vest or become exercisable upon a corporate change in control (i.e., upon a “single trigger”), butawards will vest or become exercisable in full immediately prior to an involuntary employment action that occurs within two years following a corporate change in control (i.e., upon a “double trigger”).
In the event of a corporate transaction, we can either cause the surviving corporation to assume all equity awards or accelerate their vesting and exercisability immediately before the corporate transaction. If the equity awards are assumed and an executive officer’s employment is terminated in an involuntary employment action within two years following the corporate transaction, the equity awards that are assumed will become fully vested and, if applicable, exercisable. Under our equity plans, any assumed awards that become vested will remain exercisable through the earlier of twelve12 months from the termination date or the original option expiration date. If the holder of an equity award retires, which is defined under our equity plans as leaving the employment of Biogen after reaching age 55 with at least ten10 consecutive years of service, each then outstanding time-based equity award or earned performance-based equity award not yet vested or exercisable will become immediately vested or exercisable upon such termination at a rate of 50% of the shares unvested at the time of retirement plus an additional 10% of the shares for each full year of service beyond ten10 years of service.service (and performance-based awards would remain eligible to vest based on actual performance). Options vested under these provisions remain exercisable for 36 months from retirement or until the original option expiration date, if sooner. Upon a termination of employment due to death or disability, all unvested time-based equity awards and earned performance-based equity awards vest in full and all unearned performance-based equity awards remain eligible to vest based on actual performance. | | | | | 5259 | |  | |  |
| | | | 5 | | Executive Compensation Matters (continued) |
Potential Post-Termination Payments Table The following table summarizes the potential payments to each NEO under various termination events. The table assumes that the event occurred on December 31, 2016.2018, for all NEOs. The calculations use the closing price of our common stock as reported by NASDAQ on December 30, 2016, the last business day of 2016, which was $283.58 per share. Amounts for Mr. Vounatsos are determined under our executive severance plans described above, since his employment agreement did not become effective until January 2017. Amounts for Dr. Scangos assume a terminationNasdaq on December 31, 2016 in accordance with SEC rules. On January 6, 2017, Dr. Scangos ceased to be our Chief Executive Officer and received the benefits described in the CD&A under the heading “2016 and 2017 Hiring- and Transition-Related Compensation Decisions — Dr. Scangos’ Arrangements.”2018, which was $300.92 per share. | | Name and Payment Elements(1) (a) | | Retirement(2)
(b) | | | Involuntary by the
Company Without
Cause and Not Following a Corporate
Transaction or
Change in Control
(c) | | | Involuntary
Employment
Action Following
a Corporate
Transaction or
Change in Control
(d) | | | Retirement(2) (b) | | Qualifying Termination of Employment Not Following a Corporate Transaction or Change in Control (c)(3) | | Qualifying Termination of Employment Following a Corporate Transaction or Change in Control (d)(3) | George A. Scangos(3) | | | | | | | | Severance | | | — | | | $ | 7,200,000 | | | $ | 7,200,000 | | | Performance-based RSUs(4) | | $ | 25,741,046 | | | $ | 25,741,046 | | | $ | 25,741,046 | | | Medical, Dental and Vision | | | — | | | $ | 27,110 | | | $ | 27,110 | | | Outplacement(5) | | | — | | | $ | 38,000 | | | $ | 38,000 | | | Total | | $ | 25,741,046 | | | $ | 33,006,156 | | | $ | 33,006,156 | | | Michel P. Vounatsos | | | | | | | | Michel Vounatsos(4) | | | | | | | | | | | Severance | | | — | | | $ | 1,275,000 | | | $ | 2,550,001 | | | | | — | | | | $ | 4,680,000 | | | | $ | 6,240,000 | | Performance-based RSUs | | | — | | | | — | | | $ | 3,344,749 | | | | | — | | | | | — | | | | $ | 20,523,378 | | Medical, Dental and Vision | | | — | | | $ | 19,879 | | | $ | 39,757 | | | | | — | | | | $ | 29,709 | | | | $ | 39,613 | | Outplacement(5) | | | — | | | $ | 38,000 | | | $ | 38,000 | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | Total | | | — | | | $ | 1,332,879 | | | $ | 5,972,507 | | | | | — | | | | $ | 4,741,709 | | | | $ | 26,834,991 | | Paul J. Clancy | | | | | | | | Jeffrey D. Capello | | | | | | | | | | | Severance | | | — | | | $ | 2,559,898 | | | $ | 2,925,598 | | | | | — | | | | $ | 1,487,500 | | | | $ | 2,550,000 | | Performance-based RSUs | | $ | 6,614,090 | | | $ | 6,614,090 | | | $ | 6,614,090 | | | | | | | | — | | | | $ | 2,736,395 | | Medical, Dental and Vision | | | — | | | $ | 34,080 | | | $ | 38,949 | | | | | — | | | | $ | 22,611 | | | | $ | 38,761 | | Outplacement(5) | | | — | | | $ | 38,000 | | | $ | 38,000 | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | 280G TaxGross-Up(6) | | | — | | | | — | | | | — | | | Total | | $ | 6,614,090 | | | $ | 9,246,068 | | | $ | 9,616,637 | | | | | — | | | | $ | 1,542,111 | | | | $ | 5,357,156 | | John G. Cox(7)(8) | | | | | | | | Michael D. Ehlers | | | | | | | | | | | Severance | | | | | — | | | | $ | 1,890,613 | | | | $ | 2,835,920 | | Performance-based RSUs | | | | | — | | | | | — | | | | $ | 7,240,328 | | Time-based RSUs | | | | | — | | | | | — | | | | $ | 1,736,609 | | Medical, Dental and Vision | | | | | — | | | | $ | 25,574 | | | | $ | 38,361 | | Outplacement(5) | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | Total | | | | | — | | | | $ | 1,948,187 | | | | $ | 11,883,218 | | Susan H. Alexander | | | | | | | | | | | Severance | | | — | | | $ | 2,386,967 | | | $ | 2,386,967 | | | | | — | | | | $ | 2,228,802 | | | | $ | 2,547,202 | | Performance-based RSUs | | | — | | | | — | | | $ | 7,068,736 | | | | $ | 4,671,689 | | | | $ | 4,671,689 | | | | $ | 6,673,841 | | Time-based RSUs | | | — | | | | — | | | $ | 2,394,550 | | | | $ | 852,055 | | | | $ | 852,055 | | | | $ | 1,217,221 | | Medical, Dental and Vision | | | — | | | $ | 34,080 | | | $ | 38,949 | | | | | — | | | | $ | 23,544 | | | | $ | 26,908 | | Outplacement(5) | | | — | | | $ | 38,000 | | | $ | 38,000 | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | 280G TaxGross-Up(6) | | | — | | | | — | | | | — | | | | | — | | | | | — | | | | | — | | Total | | | — | | | $ | 2,459,047 | | | $ | 11,927,202 | | | | $ | 5,523,744 | | | | $ | 7,808,090 | | | | $ | 10,497,172 | | Michael D. Ehlers(9) | | | | | | | | Paul F. McKenzie | | | | | | | | | | | Severance | | | — | | | $ | 1,317,500 | | | $ | 2,635,000 | | | | | — | | | | $ | 1,436,926 | | | | $ | 2,155,389 | | Performance-based RSUs | | | — | | | | — | | | $ | 2,586,555 | | | | | — | | | | | — | | | | $ | 5,463,914 | | Time-based RSUs | | | — | | | | — | | | $ | 860,382 | | | | | — | | | | | — | | | | $ | 1,688,161 | | Medical, Dental and Vision | | | — | | | $ | 19,475 | | | $ | 38,949 | | | | | — | | | | $ | 26,183 | | | | $ | 39,274 | | Outplacement(5) | | | — | | | $ | 38,000 | | | $ | 38,000 | | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | | Total | | | — | | | $ | 1,374,975 | | | $ | 6,158,886 | | | | | — | | | | $ | 1,495,109 | | | | $ | 9,378,738 | |
Notes to the Potential Post-Termination Payments Table | (1) | In the event of an executive’s death or disability, all outstanding time-based equity awards and earned performance-based equity awards under the Company’s LTI program will vest in full.full and all unearned performance-based equity awards will remain outstanding and eligible to vest based on actual performance. The value of such accelerated awards for all NEOs other than Dr. Scangos would be the same amount as shown in column (d) for such NEO (based on actual performance estimated as of December 31, 2016)2018). The value of Dr. Scangos’ accelerated awards would be $23,703,474, which, pursuant to his employment agreement, is calculated at the target level of performance. The grants underlying these calculations were subsequently adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of Bioverativ on February 1, 2017. The amounts reported in this table do not reflect such anti-dilution adjustments. |
| (2) | Dr. Scangos and Mr. Clancy wereMs. Alexander was eligible for potential payments upon retirement at December 31, 2016. Under Dr. Scangos’ employment agreement, upon2018. Upon retirement, all70% of his outstanding awards under the Company’s LTI program will continue to vest as if he had remained employed by the Company for the duration of the vesting period, subject to the achievement of any applicable performance criteria, and all awards that require exercise by him will remain exercisable until the earlier of three years after retirement or the original expiration date. Upon Mr. Clancy’s retirement, any vested CSPU awards would be paid to him following, if applicable, thesix-month delay required by Section 409A of the Internal Revenue Code, 70% of any unvested CSPU awards would vest immediately upon
|
| | | | | 53 | |  | |  |
| | | 5 | | Executive Compensation Matters (continued) |
| certification of the achievement of the applicable performance criteriagoals and would be paid to him following, if applicable, thesix-month delay required by Section 409A of the Internal Revenue Code,Code. Any unvested PSU and any unvested MSU awards would, subject to the achievement of any applicable performance criteria,goals, remain outstanding and vest in accordance with the terms of such awards based on actual performance as to 70% of the earned shares. The amount listed in column (b) is the estimated value of 70% of all unvested awards held by Ms. Alexander, based on actual performance estimated as of December 31, 2018, for unearned performance-based awards. Based on years of service, Ms. Alexander was eligible for accelerated vesting on 70% of her outstanding awards as of December 31, 2018. |
| (3) | The amounts listed for Dr. Scangos and Mr. Clancy in column (b) assumes(c) and column (d) for Performance-based RSUs for the applicable named executive officers includes the value of allapplicable unvested awards based on actual performance estimated as of December 31, 2016. |
(3) | On January 6, 2017, Dr. Scangos ceased to be our Chief Executive Officer and became entitled to the payments in column (c), as further described in the CD&A under the heading “2016 and 2017 Hiring- and Transition-Related Compensation Decisions — Dr. Scangos’ Arrangements.”2018. |
| (4) | Under Dr. Scangos’Pursuant to his employment agreement, inupon an involuntary termination by the case of anCompany without cause or involuntary employment action or retirement, all of his outstanding awards under the Company’s LTI program would continue to vest as if he remained employed by the Company for the duration of the vesting period, subject to the achievement of any applicable performance criteria, and all awards that require exercise by him will remain exercisable for three years (or, if earlier, until the original expiration date). The amounts listed for Dr. Scangos in columns (c) and (d) assumes the value of all unvested LTI awards based on actual performance estimated as of December 31, 2016. The actual value that will be earned, if any, will not be known until the end of the applicable performance period. |
(5) | The NEOs are also provided executive-level outplacement services at a cost of $38,000 at the executive vice president level. |
(6) | The payments for Mr. Clancy and Mr. Cox uponfollowing a corporate transaction or a corporate change in control on December 31, 2016 would not have been subject to a Section 280G excise tax. |
(7) | Biogen entered into an offer letter agreement withCIC, Mr. Cox to become the CEO of Bioverativ effective May 19, 2016. The agreement included the following enhanced severance benefits: (i) 24 months of severance instead of 21 months of severance for an involuntary termination, other than “for cause” and (2) the vesting in full of all of his outstanding awards under the Company’s LTI program in the event thespin-off of Bioverativ did not occur and the Executive Vice President, Pharmaceutical Operations and Technology and Global Therapeutic Operations position was no longer available. |
(8) | Mr. Cox voluntarily separated from the Company on January 31, 2017 in connection with the closing of the Bioverativspin-off. Mr. Cox did not receive any severance benefits in connection with such separation; however, all of Mr. Cox’s outstanding LTI awards were converted into time-based RSUs for Bioverativ common stock and will continue to vest in accordance with the service requirements of each original award. |
(9) | Dr. Ehlers would be entitled to the payments in column (d) in connection with a termination as discussed above in the section entitled “Potential Payments Upon Termination or Change in Control — Executive Severance Policy — Dr. Ehlers’ Additional Arrangements.” |
| | | | | 54 | |  | |  |
| | | 6 | | Other Management Proposals |
| | | | | | | | | | | | Proposal 4 – Advisory Vote on the Frequency of the Advisory Vote on Executive Compensation
| | | | | | | |
Proposal 3 above requests that you cast an advisory vote for the compensation disclosed in this Proxy Statement that we paid in 2016 to our named executive officers. That advisory vote is referred to as a“say-on-pay” vote. In this Proposal 4, as required pursuant to Section 14A of the Securities Exchange Act, our Board of Directors is asking that stockholders cast anon-binding, advisory vote on how frequently we should havesay-on-pay votes in the future. You can vote to holdsay-on-pay votes every one, two, or three years, or you can abstain from voting.
Our Board of Directors believes thatsay-on-pay votes should be held annually to give stockholders the opportunity to provide regular input on our executive compensation programs and increase our Board’s accountability for its compensation decisions and therefore recommends that stockholders vote for theone-year option. This vote, like thesay-on-pay vote itself, isnon-binding. If a choice other than one year receives the most votes, our Board will take the voting results into consideration in determining how frequently we will present you with asay-on-pay vote.
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THEONE-YEAR OPTION AS THE FREQUENCY OF THE ADVISORY VOTE ON EXECUTIVE COMPENSATION.
| | | | | | | | | | | | Proposal 5 – Approval of the Biogen Inc. 2017 Omnibus Equity Plan
| | | | | | | |
We are asking stockholders to approve the Biogen Inc. 2017 Omnibus Equity Plan (the 2017 Plan). Our Board of Directors, upon the recommendation of our Compensation Committee, approved the 2017 Plan, subject to stockholder approval. The 2017 Plan will not become effective unless and until it is approved by our stockholders. If the 2017 Plan is approved by our stockholders, we will no longer make grants under our 2008 Omnibus Equity Plan (the 2008 Plan). The material features of the 2017 Plan are described under “Summary of the 2017 Plan” below.
Our Board believes that the 2017 Plan will promote the interests of our stockholders and is consistent with principles of good corporate governance, including the following:
• | | Fungible Share Design. Each stock option and stock appreciation right (SAR) granted under the 2017 Plan will be counted against the share pool as one share and each other equity award will be counted against the share pool as 1.5 shares. |
• | | No Liberal Share-Recycling. Shares underlying stock options and other awards delivered under the 2017 Plan will not be recycled into the share pool if they are withheld in satisfaction of tax withholding obligations or the exercise or purchase price of the award. |
• | | Limitations on Awards. The 2017 Plan limits the number of stock options, SARs, and other awards that may be granted to plan participants in any calendar year. |
• | | Performance Awards. Under the 2017 Plan, our Compensation Committee may grant performance-based awards intended to qualify as exempt performance-based compensation under Section 162(m), as well as other performance-based awards. |
• | | No Discounted Stock Options or SARs. All stock options and SARs granted under the 2017 Plan must have a per share exercise price or base value thatVounatsos is not less than the fair market value of the underlying shares on the date of grant. |
• | | No Repricing. Other than in connection with certain corporate transactions or changes to our capital structure, the 2017 Plan prohibits the repricing of stock options or SARs without obtaining stockholder approval. |
• | | No Single-Trigger Vesting upon a Change in Control.The 2017 Plan does not provide for the automatic acceleration of equity awards in connection with a change in control. |
Reasons for Seeking Stockholder Approval
Our Board believes that equity awards have been, and will continue to be, a critical part of our total compensation program and allow us to attract and retain the key talent needed to effectively compete in our industry, incentivize superior results and long-term value creation, and align the interests of our employees with those of our stockholders. Unlike many companies, we have a practice of granting
| | | | | 55 | |  | |  |
| | | 6 | | Other Management Proposals (continued) |
equity awards to all of our employees, believing that our business will succeed if our employees are invested in us and in our future. In addition, we believe that it fosters an ownership mindset by allowing employees to take part in the successes of the Company. Our employees generally receive annual equity awards based on their individual performance and expected future contributions.
As of March 31, 2017, there were 5,867,986 shares available for grant under the 2008 Plan. Our three-year average burn rate — the number of shares granted in each year divided by the weighted shares of our common stock outstanding atyear-end — is 0.35%. We believe that our historical burn rate is reasonable in our industry, especially given our broad-based use of equity awards to compensate our employees. We will continue to monitor our equity use in future years to ensure our burn rate is within competitive market norms. Based on a review of the remaining shares available for grant under the 2008 Plan, the number of equity awards outstanding under (a) the 2008 Plan, (b) the Company’s 2005 Omnibus Equity Plan (under which we cannot grant additional awards but under which certain awards remain outstanding) (the 2005 Plan), and (c) the Company’s 2006Non-Employee Directors Equity Plan (the Directors Plan) (under whichnon-employee directors of the Company are eligible to receive equity awards), our historic burn rate, current and proposed plan features, and the equity plan guidelines established by proxy advisory firms, FW Cook supported and our Board approved the 2017 Plan and the share pool authorized under it, as described below.
In addition, stockholder approval of the 2017 Plan will allow us to continue to grant, if desired, performance-based compensation that is exempt from the deduction limitations under Section 162(m). Section 162(m) generally provides that compensation paid by a publicly-held corporation to its “covered employees” (the corporation’s chief executive officer and any of its three other named executive officers (other than its chief executive officer or chief financial officer)) is not deductible by the corporation for U.S. federal
income tax purposes for any taxable year to the extent it exceeds $1.0 million. This limitation does not apply to compensation that qualifies as exempt performance-based compensation by meeting certain requirements under Section 162(m), including the requirement that the material terms of the related performance goals be disclosed to and approved by the corporation’s stockholders not less frequently than every five years. Under Section 162(m), the material terms include the class of eligible employees, a description of the business criteria on which the performance goals may be based and the maximum amount that can be paid to any participant for a specified period. For the 2017 Plan, these terms are described below under “Eligibility,” “Performance Criteria,” and “Individual Limits,” respectively. Although stockholder approval is one of the requirements for exemption under Section 162(m), even with stockholder approval, there can be no guarantee that compensation will be treated as exempt performance-based compensation. Furthermore, our Compensation Committee will continue to have authority to provide compensation that is not exempt from the limits on deductibility under Section 162(m).
If the 2017 Plan is not approved by our stockholders, the 2017 Plan will not become effective and we will continue to grant equity awards under the 2008 Plan until the 2008 Plan expires on June 19, 2018. We believe that the terms of the 2017 Plan, including its share pool, are reasonable, appropriate, and in the best interests of our stockholders.
Existing Equity Plan Information
The 2008 Plan is the only current plan of the Company under which equity awards may be granted to our employees. As of March 31, 2017, 5,867,986 shares were available for grant under the 2008 Plan. If the 2017 Plan is approved by our stockholders, we will cease granting awards under the 2008 Plan and the 2017 Plan will be our only equity plan under which we may grant future equity awards to our employees.
| | | | | 56 | |  | |  |
| | | 6 | | Other Management Proposals (continued) |
The table below includes aggregated information regarding awards outstanding under the 2008 Plan, the 2005 Plan, and the Directors Plan, the number of shares available for future awards under each of the 2008 Plan and the Directors Plan as of March 31, 2017, and the proposed number of shares issuable under the 2017 Plan. We also maintain atax-qualified employee stock purchase plan, pursuant to which 6,008,140 shares remain outstanding as of March 31, 2017.
| | | | | | | | | | | | Number of shares
(as of March 31,
2017)(1) | | | As a percentage of stock
outstanding (214,236,610
shares as of March 31, 2017) | | Outstanding stock options and SARs (for stock options outstanding as of March 31, 2017, the weighted average exercise price was $54.02 and the weighted average remaining contractual term was 2.03 years) | | | 45,337 | | | | 0.02 | % | Outstanding full value shares, including time-vested RSUs and performance-based awards | | | 1,386,421 | | | | 0.65 | % | Total shares subject to outstanding awards | | | 1,431,758 | | | | 0.67 | % | Total shares available for future awards under the 2008 Plan(2) | | | 5,867,986 | | | | 2.74 | % | Total shares available for future awards under the Directors Plan | | | 734,333 | | | | 0.34 | % | Total shares subject to outstanding awards, available for future awards under the 2008 Pan, and available for future awards under the Directors Plan | | | 8,034,077 | | | | 3.75 | % | Proposed shares available for future awards under the 2017 Plan(3)(4) | | | 8,000,000 | | | | 3.73 | % | Total shares outstanding under existing equity awards, available for future awards, and additional shares proposed to be reserved for issuance under the 2017 Plan | | | 16,034,077 | | | | 7.48 | % |
(1) | For purposes of the number of shares subject to outstanding awards under the 2008 Plan, each share subject to a stock option or SAR is counted as one share and each share subject to any other award is counted as 1.5 shares. |
(2) | We will cease granting new awards under the 2008 Plan if the 2017 Plan is approved by our stockholders and, as described below under “Authorized Shares,” the shares remaining available for issuance under the 2008 Plan will be available for issuance under the 2017 Plan. |
(3) | For purposes of determining shares available under the 2017 Plan, each share subject to a stock option or SAR will count as one share and each share subject to any other award will count as 1.5 shares. Because the 2017 Plan does not specify a mix of stock options and SARs, on the one hand, and other awards, on the other, it is not possible to determine the amount of subsequent dilution that may ultimately result from such awards. Other share-counting provisions, including adjustments to the numbers of shares available under the 2017 Plan, are described below under “Authorized Shares.” |
(4) | Subject to adjustment as described below under “Authorized Shares,” the maximum number of shares of our common stock that may be delivered in satisfaction of awards under the 2017 Plan is 8,000,000 plus any shares of stock that either remain available for grant as of the date of adoption of the 2017 Plan (including shares available by reason of a predecessor plan) or are subject to awards under the 2008 Plan and on or after the date of adoption of the 2017 Plan are cancelled, surrendered, exchanged, terminated, or forfeited for any reason in accordance with the terms of such plan. |
Summary of the 2017 Plan
The following is a brief summary of the material features of the 2017 Plan. A copy of the 2017 Plan is attached as Appendix B to this Proxy Statement, and we urge stockholders to read it in its entirety. The following summary is qualified in its entirety by reference to the full text of the 2017 Plan.
Administration.The 2017 Plan is administered by our Compensation Committee, which has the discretionary authority to, among other things, interpret the 2017 Plan, determine eligibility for and grant awards, determine, modify or waive the terms and conditions of any award, determine the form of settlement of awards, prescribe forms, rules and procedures for awards, and otherwise do all things necessary or desirable to carry out the purposes of the 2017 Plan. Determinations of our Compensation Committee under the
2017 Plan will be conclusive and bind all parties. Our Board may perform any of the functions of our Compensation Committee under the 2017 Plan, and our Compensation Committee may delegate such of its duties, powers, and responsibilities as it may determine. As used herein, the term “Compensation Committee” refers to our Board, our Compensation Committee, or its authorized delegates, as applicable.
Eligibility.All employees of the Company and its affiliates are eligible to participate in the 2017 Plan. As of March 31, 2017 we had approximately 7,100 employees.
Authorized Shares.Subject to adjustment as described below, the maximum number of shares of our common stock that may be delivered in satisfaction of awards under the 2017 Plan is 8,000,000, plus any shares of stock that either remain available for grant as of the date of adoption
| | | | | 57 | |  | |  |
| | | 6 | | Other Management Proposals (continued) |
of the 2017 Plan (including shares available by reason of a predecessor plan) or are subject to awards under the 2008 Plan and on or after the date of adoption of the 2017 Plan are cancelled, surrendered, exchanged, terminated, or forfeited for any reason in accordance with the terms of such plan (the Share Pool). Up to 1,000,000 shares from the Share Pool may be issued as stock options intended to qualify as incentive stock options (ISOs). For purposes of the Share Pool:
Each share subject to an award of stock options or SARs will reduce the Share Pool by one share and each share subject to any other award will reduce the Share Pool by 1.5 shares.
All shares covering a SAR, any portion of which is settled in stock, will reduce the Share Pool.
Shares withheld in satisfaction of tax withholding obligations or the exercise or purchase price of an award will not return to the Share Pool.
The Share Pool will not be reduced to the extent any portion of an award is settled in cash or property (other than shares).
If an award expires or is terminated or cancelled without having been exercised or settled in full, or if shares acquired pursuant to an award subject to forfeiture or repurchase are forfeited or repurchased by the Company, or if shares subject to an award under the 2008 Plan return to the Share Pool, the shares allocable to the terminated portion of such award or such forfeited or repurchased shares, or the shares subject to such award under the 2008 Plan, will return to the Share Pool at the rate of one share for each share subject to an award of stock options or SARs and 1.5 shares for each share subject to any other award.
Shares issued under awards granted by another company and assumed orsubstituted-for by the Company will not reduce the Share Pool.
Shares that may be delivered under the 2017 Plan may be authorized but unissued shares or treasury shares. The closing price of our common stock as reported on NASDAQ on March 31, 2017 was $273.42 per share.
Individual Limits.The maximum number of shares subject to awards that may be granted to any participant in any calendar year is 1,500,000 shares in the aggregate, subject to adjustment as described below.
Types of Awards.The 2017 Plan provides for the grant of stock options, SARs, RSUs, restricted stock awards (RSAs),
and other awards (including performance awards). Dividends, dividend equivalents, or other cash payments may also be provided in connection with awards under the 2017 Plan.
• | | Stock Options and SARs.Our Compensation Committee may grant stock options, including ISOs and SARs. A stock option is a right entitling the holder to acquire shares of our common stock upon payment of the applicable exercise price. A SAR is a right entitling the holder upon exercise to receive an amount (payable in cash or shares or other property of equivalent value) equal to the excess of the fair market value of the shares subject to the right over the base value from which appreciation is measured. The exercise price of each stock option, and the base value of each SAR, granted under the 2017 Plan shall be no less than 100% of the fair market value of a share of our common stock on the date of grant (110% in the case of certain ISOs). Other than adjustments in connection with certain corporate transactions or changes to our capital structure as described below, stock options and SARs granted under the 2017 Plan may not be amended to reduce the exercise price or base value or cancelled in exchange for stock options or SARs with a lower exercise price or base value, nor may any consideration be paid upon the cancellation of any stock options or SARs that have a per share exercise price or base value greater than the fair market value of a share of our common stock on the date of such cancellation, in each case, without stockholder approval. The expiration date of each stock option and SAR granted under the 2017 Plan shall be ten years from the date of grant, or such earlier time as our Compensation Committee may determine, and vested awards that expire are automatically exercised on the expiration date if the per share exercise price or base value is less than the fair market value of a share on that date. |
• | | RSUs and RSAs.Our Compensation Committee may grant awards of RSUs or RSAs. An RSU is an unfunded and unsecured promise, denominated in shares, to deliver shares or cash measured by the value of shares in the future, subject to the satisfaction of specified performance or other vesting conditions. An RSA is stock subject to restrictions requiring that it be redelivered or offered for sale to us if specified service or performance-based conditions are not satisfied. |
• | | Performance Awards.Our Compensation Committee may grant performance awards, which are awards subject to performance criteria. Our Compensation Committee may grant performance awards that are intended tolump sum payment within 60 days
|
| | | | | 58 | |  | |  |
| | | 6 | | Other Management Proposals (continued) |
| | qualify as exempt performance-based compensation under Section 162(m) and awards that are not intended to so qualify.
|
• | | Other Awards.Our Compensation Committee may grant other awards convertible into or otherwise based on shares, subject to such terms and conditions as it may determine. |
Vesting; Terms of Awards.Our Compensation Committee determines the terms of all awards granted under the 2017 Plan and may impose such restrictions or conditions to vesting as it deems appropriate, including requiring the achievement of performance criteria. Awards under the 2017 Plan that vest solely based on continued employment shall vest on a schedule that provides for vesting in equal installments on each of the first three anniversaries of the date of grant, or such longer period as our Compensation Committee may determine, except that up to 500,000 shares of stock may be subject to awards that provide for vesting sooner than this schedule and awards will be subject to accelerated vesting in connection with certain terminations of employment, as described below, or as set forth in a participant’s award agreement.
Transferability of Awards.Awards may not be transferred other than by will or by the laws of descent and distribution, except that our Compensation Committee may permit stock options other than ISOs to be transferred to family members of a participant or to certain entities controlled by the participant or his or her family members.
Performance Criteria.The 2017 Plan provides for grants of performance awards subject to “performance criteria.” Performance criteria with respect to those awards that are intended to qualify as “performance-based compensation” for purposes of Section 162(m) are limited to objectively determinable measure(s) of performance relating to any or any combination of the following (measured either absolutely or comparatively and on a consolidated or other basis): sales; revenues; assets; expenses; earnings before or after deduction for all or any portion of interest, taxes, depreciation, or amortization or other items, whether or not on a continuing operations or an aggregate or per share basis; return on equity, investment, capital, or assets; one or more operating ratios; borrowing levels, leverage ratios, or credit rating; market share; capital expenditures; cash flow; stock price; stockholder return; sales of particular products or services; customer acquisition, expansion, or retention;
acquisitions and divestitures (in whole or in part); joint ventures and strategic alliances; spin-offs,split-ups, and the like; reorganizations; recapitalizations, restructurings, financings (issuance of debt or equity), or refinancings; or achievement of clinical trials or measurable research objectives.
Performance criteria and any related targets need not be based on an increase, a positive or improved result, or avoidance of loss and may be based on GAAP,non-GAAP, or other metrics. To the extent consistent with the requirements of Section 162(m), our Compensation Committee may establish that one or more of the applicable performance criteria will be adjusted in an objectively determinable manner to reflect events (for example, acquisitions or dispositions) occurring during the applicable performance period that affect the applicable performance criteria.
Effect of Termination of Employment.Unless an award agreement expressly provides otherwise, immediately upon the termination of a participant’s employment, unvested awards will be forfeited and awards requiring exercise will cease to be exercisable, except that:
If the termination of employment is due to the participant’s death or disability, each award will become fully vested and each award requiring exercise will remain exercisable for one year (or the remainder of the original term, if less);
If the termination is due to the participant’s retirement (generally, a voluntary termination of employment after attaining age 55 with ten years of employment with the Company and its affiliates), each award will become vested as to 50% of the shares subject thereto, plus an additional 10% of such shares for each full year of consecutive employment in excess of ten years, provided that awards subject to performance criteria will so vest only to the extent consistent with the requirements of Section 162(m), and (iii) each award requiring exercise, to the extent then exercisable, will remain exercisable for three years (or the remainder of the original term, if less);
If the termination of employment is for cause, each award will terminate on the date of such termination; and
If the termination of employment is for any other reason, each award requiring exercise, to the extent then exercisable, will remain exercisable for six months (or the remainder of the original term, if less).
| | | | | 59 | |  | |  |
| | | 6 | | Other Management Proposals (continued) |
Effect of Certain Transactions.Unless an award agreement expressly provides otherwise, in the event of a corporate change in control or any other consolidation, merger, or similar transaction in which the Company is not the surviving corporation, a sale of all or substantially all of the Company’s assets, or a dissolution or liquidation of the Company:
If there is an acquiring or surviving entity, our Compensation Committee shall provide for the assumption, substitution, or continuation of some or all awards;
If at any time within two years thereafter the participant’s employment is terminated without cause or the participant resigns under certain circumstances, any awards that are assumed, substituted for, or continued in connection with such transaction will vest in full;
If stockholders will receive a payment in the transaction, our Compensation Committee may provide for a payout of some or all awards on such payment and other terms and conditions as it may determine; and
Awards that are not assumed, substituted for, or continued will terminate upon the consummation of the transaction.
Adjustment Provisions.In the event of a stock dividend or similar distribution, stock split or combination of shares (including a reverse stock split), recapitalization, or other change in our capital structure, our Compensation Committee shall make appropriate adjustments to the maximum number of shares that may be delivered under the 2017 Plan; the individual award limits; the number and kind of securities subject to, and, if applicable, the exercise price or base value of, outstanding awards; and any other provisions affected by such event. Our Compensation Committee may make similar adjustments for other events if it determines adjustments are appropriate to avoid distortion in the operation of the 2017 Plan or to preserve the value of awards.
Clawback.Our Compensation Committee may cancel, rescind, withhold, or otherwise limit or restrict awards if a participant is not in compliance with the provisions of the 2017 Plan or engages in certain detrimental activities. In addition, our Compensation Committee may provide that awards and related proceeds are subject to forfeiture or disgorgement to the extent required or permitted by applicable Company policy, law, or stock exchange listing standards.
Effective Date,Amendments and Termination.If the 2017 Plan is approved by our stockholders, the 2017 Plan will become effective as of the date of such approval. No
awards will be granted after the tenth anniversary of such approval. Our Compensation Committee may at any time amend, suspend, or terminate the 2017 Plan or any portion thereof, subject to such stockholder approval as our Compensation Committee determines necessary or advisable, except that no such amendment, suspension, or termination will adversely affect the rights of a participant under a previously granted award (without the participant’s consent) and no amendment will effectuate a change for which stockholder approval is required without obtaining such approval.
Certain Federal Income Tax Consequences
The following is a summary of certain U.S. federal income tax consequences associated with certain awards granted under the 2017 Plan. The summary does not purport to cover federal employment tax or other U.S. federal tax consequences that may be associated with the 2017 Plan, nor does it cover state, local, ornon-U.S. taxes, except as may be specifically noted.
Stock Options (other than ISOs).In general, a participant has no taxable income upon the grant of a stock option that is not intended to be an ISO (an NSO) but realizes income in connection with the exercise of the NSO in an amount equal to the excess (at the time of exercise) of the fair market value of the shares acquired upon exercise over the exercise price. A corresponding deduction is generally available to the Company. Upon a subsequent sale or exchange of the shares, any recognized gain or loss is treated as a capital gain or loss for which the Company is not entitled to a deduction.
ISOs.In general, a participant realizes no taxable income upon the grant or exercise of an ISO. However, the exercise of an ISO may result in an alternative minimum tax liability to the participant. Generally, a disposition of shares purchased pursuant to an ISO within two years from the date of grant or within one year after exercise produces ordinary income to the participant (and generally a deduction to the Company) equal to the value of the shares at the time of exercise less the exercise price. Any additional gain recognized in the disposition is treated as a capital gain for which the Company is not entitled to a deduction. If the participant does not dispose of the shares until after the expiration of theseone- andtwo-year holding periods, any gain or loss recognized upon a subsequent sale of shares purchased pursuant to an ISO is treated as a long-term capital gain or loss for which the Company is not entitled to a deduction.
SARs.The grant of a SAR does not itself result in taxable income, nor does taxable income result merely because a
| | | | | | 60 | |  | |  |
| | | 65 | | Other Management ProposalsExecutive Compensation Matters (continued)
|
| consisting of the pro rata portion of the target bonus for the year of termination and an amount equal to the sum of the annual base salary rate and target bonus in effect at the time of termination multiplied by a factor of 1.5, continuation of medical, dental and vision insurance for up to 18 months and up to 12 months of executive outplacement services. Upon an involuntary termination by the Company without cause or an involuntary employment action following a corporate transaction or CIC, Mr. Vounatsos is eligible to receive a lump sum payment within 60 days consisting of the pro rata portion of the target bonus for the year of termination and an amount equal to the sum of the annual base salary rate and target bonus in effect at the time of termination multiplied by a factor of 2.0, continuation of medical, dental and vision insurance for up to 24 months and up to 12 months of outplacement services. |
| (5) | The named executive officers are provided outplacement services at a cost of up to $32,000 for the Executive Vice President level. |
| (6) | The payments for Ms. Alexander upon a corporate transaction or a corporate change in control on December 31, 2018, would not have been subject to a Section 280G excise tax. |
CEO Pay Ratio We believe executive pay must be internally consistent and equitable to motivate our employees to create stockholder value and we are committed to internal pay equity. As discussed earlier in this Proxy Statement, our compensation programs are designed to drive the creation of long-term stockholder value by delivering performance-based compensation. We invest in our employees at all levels in the Company by rewarding performance that balances risk and reward, empowering professional growth and development and by offering affordable benefits and programs that meet the diverse needs of our employees. SAR becomes exercisable.We believe strongly inpay-for-performance and all of our employees are eligible to participate in our annual bonus plan, our LTI programs and our benefit plans. Our annual bonus plan is consistently maintained for all participants globally, with the same Company performance goals, payout levels (as a percentage of target) and administrative provisions regardless of the participant’s job level, location or function in the Company. We also have a long-term incentive program that provides different forms of awards depending upon an employee’s level but is otherwise consistent throughout the Company.
The following is a reasonable estimate, prepared under applicable SEC rules, of the ratio of the annual total compensation of our CEO to the median of the annual total compensation of our other employees. Under SEC rules, we used the same median employee as we used for our 2017 pay ratio because we reasonably believe there have been no changes to our employee population or compensation programs that would result in a significant change to our pay ratio disclosure. The methodology used to identify our median employee for our 2017 pay ratio is described in our 2018 proxy statement. Our median employee is a full-time employee based in the U.S. In general, a participant who exercises a SAROctober 2017, when we determined the median employee, approximately 59% of our workforce was based in the U.S. with the remaining approximately 41% of our workforce based in the rest of the world. In addition, approximately 98% of our workforce was full-time. For our median employee, annual total compensation was calculated in accordance with the SEC’s rules for sharesthe Summary Compensation Table, including salary, bonus, LTI grant date fair value and value of stock or receives payment in cancellation of a SAR will have ordinary incomecertain benefits provided, including relocation benefits. For our CEO, annual total compensation is equal to the amount included in the “Total” column of any cashthe Summary Compensation Table, and our CEO’s annual total compensation for 2018 was $16,168,646. The annual total compensation of the median employee for 2018 was $170,521, including base salary, annual bonus, LTI grant value, and certain benefits includingone-time relocation benefit. Based on the foregoing, our estimate of the ratio of the annual total compensation of our CEO to the median of the annual total compensation of our other employees was 95 to 1. This pay ratio is a reasonable estimate calculated in a manner consistent with SEC rules based on our payroll and employment records and the fair market valuemethodology described above. Given the different methodologies, estimates, assumptions and exclusions that other public companies use to determine an estimate of any stock received. A corresponding deduction is generally available totheir pay ratio, the Company. RSAs.A participant who is awarded or purchases shares subject to a substantial risk of forfeiture generally doesestimated ratio reported above should not have income until the risk of forfeiture lapses. When the risk of forfeiture lapses, the participant has ordinary income equal to the excess of the fair market value of the shares at that time over the purchase price, if any, and a corresponding deduction is generally available to the Company. However, a participant may make an election under Section 83(b) of the Internal Revenue Code to be taxed on restricted stock when it is acquired rather than later, when the substantial risk of forfeiture lapses. A participant who makes an effective 83(b) election will realize ordinary income equal to the fair market value of the shares as of the time of acquisition less any price paid for the shares. A corresponding deduction will generally be available to the Company. If a participant makes an effective 83(b) election, no additional income results by reason of the lapsing of the restrictions.
For purposes of determining capital gain or loss on a sale of shares awarded under the 2017 Plan, the holding period in the shares begins when the participant recognizes taxable income with respect to the transfer. The participant’s tax basis in the shares equals the amount paid for the shares plus any income realized with respect to the transfer. However, if a participant makes an effective 83(b) election and later forfeits the shares, the tax loss realizedused as a result of the forfeiture is limited to the excess of what the participant paidbasis for the shares (if anything) over the amount (if any) realized in connection with the forfeiture.comparison between companies.
RSUs.The grant of a RSU does not itself generally result in taxable income. Instead, the participant is generally taxed
upon vesting (and a corresponding deduction is generally available to the Company), unless he or she has made a proper election to defer receipt of the shares (or cash if the award is cash settled) under Section 409A of the Internal Revenue Code. If the shares delivered are restricted for tax purposes, the participant will instead be subject to the rules described above for restricted stock.
Section 162(m). Stock options, SARs, and certain performance awards under the 2017 Plan may be eligible for exemption from the deductibility limits of Section 162(m). However, our Board will have discretionary authority to provide compensation that is not exempt from the limits on deductibility under Section 162(m).
Certain Change in Control Payments.Under Section 280G of the Internal Revenue Code, the vesting or accelerated exercisability of options or the vesting and payments of other awards in connection with a change in control of a corporation may be required to be valued and taken into account in determining whether participants have received compensatory payments, contingent on the change in control, in excess of certain limits. If these limits are exceeded, a substantial portion of amounts payable to the participant, including income recognized by reason of the grant, vesting, or exercise of awards may be subject to an additional 20% federal tax and may benon-deductible to the Company.
New Plan Benefits
Because awards under the 2017 Plan will be granted in the discretion of our Compensation Committee, the type, number, recipients, and other terms of such awards cannot be determined at this time.
Vote Required
The 2017 Plan will be approved upon the affirmative vote of a majority of the votes present in person or represented by proxy and having voting power at the Annual Meeting.
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTEFOR THE APPROVAL OF THE BIOGEN INC. 2017 OMNIBUS EQUITY PLAN.
| | | | | | 61 | |  | |  |
| | | 76 | | Additional Information |
STOCK OWNERSHIP The following table and accompanying notes provide information about the beneficial ownership of our common stock by: each stockholder known by us to be the beneficial owner of more than 5% of our common stock; each of our named executive officers (listed in the Summary Compensation Table);officers; each of our directors and nominees for director; and all of our directors and executive officers as a group. Except as otherwise noted, the persons identified have sole voting and investment power with respect to the shares of our common stock beneficially owned. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting and investment power with respect to the shares. Except as otherwise noted, the information below is as of April 13, 201719, 2019 (Ownership Date). Unless otherwise indicated in the footnotes, the address of each of the individuals named below is: c/o Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142. | | | | | | | | | | | | | | | | | | Name | | Shares
Owned (1) | | | Shares Subject to
Options and
Stock Units(2) | | | Total Number of
Shares Beneficially
Owned | | | Percentage of
Outstanding
Shares(3) | | BlackRock, Inc.(4) 55 East 52nd Street New York, NY 10022 | | | 17,082,146 | | | | — | | | | 17,082,146 | | | | 8.01 | % | PRIMECAP Management Company(5) 177 East Colorado Boulevard 11th Floor Pasadena, CA 91105 | | | 15,584,387 | | | | — | | | | 15,584,387 | | | | 7.31 | % | The Vanguard Group(6) 100 Vanguard Boulevard Malvern, PA 19355 | | | 14,009,922 | | | | — | | | | 14,009,922 | | | | 6.57 | % | Paul J. Clancy | | | 20,705 | | | | — | | | | 20,705 | | | | * | | John G. Cox(7) | | | 30,601 | | | | — | | | | 30,601 | | | | * | | Alexander J. Denner(8) | | | 317,890 | | | | 1,084 | | | | 318,974 | | | | * | | Caroline D. Dorsa | | | 16,033 | | | | 1,084 | | | | 17,117 | | | | * | | Michael Ehlers(9) | | | — | | | | 5,598 | | | | 5,598 | | | | | | Nancy L. Leaming | | | 7,924 | | | | 1,084 | | | | 9,008 | | | | * | | Richard C. Mulligan | | | 7,890 | | | | 1,084 | | | | 8,974 | | | | * | | Robert W. Pangia | | | 15,568 | | | | 13,030 | | | | 28,598 | | | | * | | Stelios Papadopoulos(10) | | | 26,580 | | | | 1,626 | | | | 28,206 | | | | * | | Brian S. Posner | | | 5,275 | | | | 1,084 | | | | 6,359 | | | | * | | Eric K. Rowinsky | | | 12,005 | | | | 1,084 | | | | 13,089 | | | | * | | George A. Scangos(11) | | | 82,497 | | | | — | | | | 82,497 | | | | * | | Lynn Schenk(12) | | | 7,990 | | | | 1,084 | | | | 9,074 | | | | * | | Stephen A. Sherwin | | | 3,845 | | | | 13,362 | | | | 17,207 | | | | * | | Michel Vounatsos(9) | | | 1,525 | | | | 3,244 | | | | 4,769 | | | | | | Executive officers and directors as a group (18 persons)(9)(13) | | | 492,518 | | | | 50,834 | | | | 543,361 | | | | * | |
| | | | | | | | | | | | | | | | | | | | | | | Name | | Shares
Owned(1) | | | Shares Subject to Options and Stock Units(2) | | | Total Number of Shares Beneficially Owned(1) | | | Percentage of Outstanding Shares(3) | | 5% Stockholders | | | | | | | | | | | | | | | | | BlackRock Inc.(4) 55 East 52nd Street New York, NY 10055 | | | 16,568,963 | | | | — | | | | 16,568,963 | | | | 8.2 | % | The Vanguard Group(5) 100 Vanguard Boulevard Malvern, PA 19355 | | | 15,170,607 | | | | — | | | | 15,170,607 | | | | 7.52 | % | PRIMECAP Management Company(6) 177 East Colorado Boulevard 11th Floor Pasadena, CA 91105 | | | 14,727,267 | | | | — | | | | 14,727,267 | | | | 7.31 | % | Named Executive Officers | | | | | | | | | | | | | | | | | Michel Vounatsos(7) | | | 16,657 | | | | 3,252 | | | | 19,909 | | | | * | | Jeffrey D. Capello | | | 917 | | | | — | | | | 917 | | | | * | | Michael Ehlers(7) | | | 6,824 | | | | 3,538 | | | | 10,362 | | | | * | | Susan H. Alexander | | | 31,976 | | | | — | | | | 31,976 | | | | * | | Paul F. McKenzie | | | 6,407 | | | | — | | | | 6,407 | | | | * | | Directors | | | | | | | | | | | | | | | | | John R Chiminski | | | — | | | | — | | | | — | | | | — | | Alexander J. Denner(8) | | | 534,687 | | | | 880 | | | | 535,567 | | | | * | | Caroline D. Dorsa | | | 18,172 | | | | 880 | | | | 19,052 | | | | * | | William A. Hawkins | | | — | | | | — | | | | — | | | | — | | Nancy L. Leaming | | | 10,063 | | | | 880 | | | | 10,943 | | | | * | | Jesus B. Mantas | | | — | | | | — | | | | — | | | | — | | Richard C. Mulligan | | | 10,029 | | | | 880 | | | | 10,909 | | | | * | | Robert W. Pangia | | | 17,707 | | | | 880 | | | | 18,587 | | | | * | | Stelios Papadopoulos(9) | | | 29,946 | | | | 1,450 | | | | 31,396 | | | | * | | Brian S. Posner | | | 6,015 | | | | 880 | | | | 6,895 | | | | * | | Eric K. Rowinsky | | | 14,144 | | | | 880 | | | | 15,024 | | | | * | | Lynn Schenk(10) | | | 10,097 | | | | 880 | | | | 10,977 | | | | * | | Stephen A. Sherwin | | | 4,284 | | | | 13,158 | | | | 17,442 | | | | * | | Executive officers and directors as a group (23 persons)(7)(11) | | | 731,239 | | | | 28,438 | | | | 759,677 | | | | * | |
| * | Represents beneficial ownership of less than 1% of our outstanding shares of common stock. |
| | | | | | 62 | |  | |  |
| | | | 6 | | Additional Information (continued) |
| (1) | The shares described as “owned” are shares of our common stock directly or indirectly owned by each listed person.person, rounded up to the nearest whole share. |
| (2) | Includes options that are or will become exercisable and RSUs and MSUs that will vest within 60 days of the Ownership Date. |
| (3) | The calculation of percentages is based upon 213,116,278193,893,397 shares outstanding on the Ownership Date, plus for each of the individuals listed above the shares subject to options and RSUs and MSUs exercisable within 60 days of the Ownership Date, as reflected in the column under the heading “Shares Subject to Options and Stock Units.” |
| | | | | 62 | |  | |  |
| | | 7 | | Additional Information (continued) |
| (4) | Based solely on information as of December 31, 20162018, contained in a Schedule 13G/A filed with the SEC by BlackRock Inc. on January 19, 2017,February 4, 2019, which also indicates that it has sole voting power with respect to 14,947,18914,695,569 shares and sole dispositive power with respect to 17,073,676 shares, and shared voting and shared dispositive power with respect to 8,47016,568,963 shares. |
| (5) | Based solely on information as of December 31, 2016 contained in Form13F-HR filed with the SEC by PRIMECAP Management Company on February 13, 2017, which also indicates that it has sole voting power over 2,054,072 shares. |
(6) | Based solely on information as of December 31, 20162018, contained in a Schedule 13G/A filed with the SEC by The Vanguard Group on February 10, 2017,11, 2019, which also indicates that it has sole voting power with respect to 340,469246,897 shares, sole dispositive power with respect to 13,632,39614,880,929 shares, shared voting power with respect to 40,24347,063 shares and shared dispositive power with respect to 377,526289,678 shares. |
| (6) | Based solely on information as of December 31, 2018, contained in a Schedule 13G/A filed with the SEC by PRIMECAP Management Company on February 8, 2019, which also indicates that it has sole voting power over 1,696,975 shares and sole dispositive power over 14,727,267 shares. |
| (7) | Mr. Cox voluntarily separated from the Company on January 31, 2017 in connection with the closing of the Bioverativspin-off. |
(8) | Includes (i) 190,142 shares of common stock directly beneficially owned by Sarissa Capital Domestic Fund LP, a Delaware limited partnership (Sarissa Domestic); and (ii) 119,858 shares of common stock directly beneficially owned by Sarissa Capital Offshore Master Fund LP, a Cayman Islands limited partnership (Sarissa Offshore and, together with Sarissa Domestic, the Sarissa Funds). Sarissa Capital Management GP LLC, a Delaware limited liability company (Sarissa Capital GP), is the general partner of Sarissa Capital Management LP, a Delaware limited partnership (Sarissa Capital), the investment advisor to the Sarissa Funds. Alexander Denner is the Chief Investment Officer of Sarissa Capital and the managing member of Sarissa Capital GP. By virtue of the foregoing, Dr. Denner may be deemed to indirectly beneficially own the shares that the Sarissa Funds directly beneficially own. Dr. Denner disclaims beneficial ownership of such shares of common stock owned by the Sarissa Funds. |
(9) | Includes shares underlying market stock unitsMSUs that will vest within 60 days of the Ownership Date, assuming the maximum possible number of shares that are eligible for vesting on the vesting date. The actual number of shares that will vest on each vesting date will be determined by comparing the price of Biogen common stock on such vesting date to the price on the grant date (i.e., number of vested shares = number of shares at target payout times the[30-day average closing stock price ending on the vesting date divided by the30-day average closing stock price on the grant date]). |
(10)(8) | Includes 10,000383,858 shares beneficially owned by Sarissa Capital Offshore Master Fund LP, a Cayman Islands exempted limited partnership (Sarissa Offshore), 79,800 shares beneficially owned by Sarissa Capital Catapult Fund LLC, a Delaware limited liability company (Sarissa Catapult) and 61,000 shares beneficially owned by Sarissa Capital Management LP, a Delaware limited partnership (Sarissa Capital). Sarissa Capital is the investment advisor to certain investment funds, including Sarissa Offshore and Sarissa Catapult. Dr. Denner is the Chief Investment Officer of Sarissa Capital and controls the ultimate general partner of each of Sarissa Capital and Sarissa Offshore and the managing member of Sarissa Catapult. By virtue of the foregoing, Dr. Denner may be deemed to indirectly beneficially own (as that term is defined in Rule13d-3 of the Exchange Act) the shares that those entities beneficially own. Dr. Denner disclaims beneficial ownership of these shares except to the extent of any pecuniary interest therein. |
| (9) | Includes 28,206 shares held in limited liability companies of which Dr. Papadopoulos is the sole manager. |
(11)(10) | Includes 10,756 shares held in trusts of which Dr. Scangos is a trustee. Dr. Scangos ceased to be Biogen’s Chief Executive Officer and a member of our Board, effective January 6, 2017. |
(12) | Includes 3,100738 shares held in a trust of which Ms. Schenk is a trustee.trustee and 2,362 shares held in a defined benefit plan. |
(13)(11) | Includes 323,100555,964 shares held indirectly through trusts, funds, defined benefit plans or limited liability companies. |
Section 16(a) Beneficial Ownership Reporting Compliance Section 16(a) of the Securities Exchange Act requires our executive officers, directors and greater than 10% stockholders to file initial reports of ownership and changes of ownership of our common stock. As a practical matter, we assist our directors and executive officers by monitoring transactions and completing and filing Section 16 forms on their behalf. Based solely on information provided to us by our directors and executive officers, we believe that during 20162018 all such parties complied with all applicable filing requirements. | | | | | | 63 | |  | |  |
| | | 76 | | Additional Information (continued) |
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS Our Code of Business Conduct (Values in Action), Corporate Governance Principles, Related Person Transaction Policy and Conflict of Interest Policy set forth our policies and procedures for the review and approval of transactions with related persons, including transactions that would be required to be disclosed in this Proxy Statement in accordance with SEC rules. In circumstances where one of our directors or executive officers, or a family member, has a direct or indirect material interest in a transaction involving Biogen, our Corporate Governance Committee must review and approve all such proposed transactions or courses of dealing. In determining whether to approve or ratify a transaction with a related person, among the factors our Corporate Governance Committee may consider (as applicable) are: the business reasons for entering into the transaction; the size of the transaction and the nature of the related person’s interest in the transaction; whether the transaction terms are as favorable to us as they would be to an unaffiliated third party; whether the transaction terms are more favorable to the related person than they would be to an unaffiliated third party; the availability of alternative sources for comparable products, services or other benefits; whether the transaction would impair the independence or judgment of the related person in the performance of his or her duties to us; fornon-employee directors, whether the transaction would be consistent with NASDAQ’sNasdaq’s requirements for independent directors; whether the transaction is consistent with our Conflict of Interest Policy, which prohibits related persons and others from having a financial interest in any competitor, customer, vendor or supplier of ours; the related person’s role in arranging the transaction; the potential for the transaction to be viewed as representing or leading to an actual or apparent conflict of interest; and any other factors that our Corporate Governance Committee deems appropriate. Other than the sponsored research agreement described below, there are no relationships or transactions with related persons that are required to be disclosed in this Proxy Statement under SEC rules. Indeed, ourOur Code of Business Conduct, which sets forth legal and ethical guidelines for all of our directors and employees, states that directors, executive officers and employees must avoid relationships or activities that might impair their ability to make objective and fair decisions while acting in their Company roles.
On January 27, 2017, we entered into a sponsored research agreement There are no relationships or transactions with Harvard University (the Research Agreement),related persons that are required to be disclosed in this Proxy Statement under which the Artavanis-Tsakonas Laboratory at Harvard Medical School will conduct research to identify novel genes, targets, and pathways that regulate neurodegenerative diseases. We believe the Research Agreement will support the identification of new drug targets and pathways in a resource efficient manner. Under the Research Agreement, we have an option to negotiate an exclusive license to any invention resulting from projects funded under the agreement. Dr. Spyros Artavanis-Tsakonas is the Principal Investigator and directs the activities of the Artavanis-Tsakonas Laboratory and is a Professor of Cell Biology at Harvard Medical School. Dr. Artavanis-Tsakonas currently serves as a Visiting Scientist at Biogen, which is a part-time position, and previously served as our Senior Vice President, Chief Scientific Officer. The Research Agreement requires us to make payments to Harvard University of $1.7 million per year, of which $1.0 million per year will directly fund the sponsored research at the Artavanis-Tsakonas Laboratory. The Research Agreement has an initial term of five years and may, after three years, be terminated on six months’ notice if certain milestones have not been met.SEC rules.
| | | | | | 64 | |  | |  |
| | | 76 | | Additional Information (continued) |
EQUITY COMPENSATION PLAN INFORMATION The following table provides information as of December 31, 20162018, about: the number of shares of common stock subject to issuance upon exercise of outstanding options and vesting of RSUs, MSUs and PSUs under plans adopted and assumed by us; the weighted-average exercise price of outstanding options under plans adopted and assumed by us; and the number of shares of common stock available for future issuance under our active plans: the 2008our 2017 Omnibus Equity Plan, the 2006ourNon-Employee Directors Equity Plan and theour 2015 Employee Stock Purchase Plan. | | Plan Category | | Number of Securities
to be Issued Upon
Exercise of
Outstanding Options
and Rights (a) | | Weighted-average Exercise Price of
Outstanding
Options and Rights(1) (b) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column(a))(2) (c) | | Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights (a) | | Weighted-average Exercise Price of Outstanding Options and Rights(1) (b) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column(a))(2) (c) | Equity compensation plans approved by stockholders | | 1,415,759 | | $ 54.06 | | 13,144,980 | | 1,308,264 | | $ 53.82 | | 20,564,534 | Equity compensation plans not approved by stockholders | | — | | — | | — | | — | | — | | — | Total | | 1,415,759 | | $ 54.06 | | 13,144,980 | | 1,308,264 | | $ 53.82 | | 20,564,534 |
| (1) | The weighted-average exercise price includes all outstanding stock options but does not include RSUs MSUs or PSUs, which do not have an exercise price. If the RSUs were included in this calculation, the weighted average exercise price would be $2.51. The total number of RSUs included in column (a) is 1,350,291. |
| (2) | Of these shares, (a) 6,496,00014,127,581 remain available for future issuance under our 20082017 Omnibus Equity Plan, (b) 717,613703,425 remain available for future issuance under our 2006Non-Employee Directors Equity Plan and (c) 5,931,3675,733,528 remain available under our 2015 Employee Stock Purchase Plan. In addition to shares issuable upon the exercise of options or rights, the shares under our 20082017 Omnibus Equity Plan and our 2006Non-Employee Directors Equity Plan may also be issued other than upon such exercise. |
| | | | | | 65 | |  | |  |
| | | 76 | | Additional Information (continued) |
MISCELLANEOUS Stockholder Proposals Stockholder proposals submitted pursuant to Securities Exchange Act Rule14a-8 and intended to be presented at our 20182020 annual meeting of stockholders must be received by our Secretary no later than December 27, 201730, 2019, to be eligible for inclusion in our proxy statement and form of proxy relating to that meeting. A stockholder proposal submitted outside the processes of Rule14a-8 and not for inclusion in our proxy statement for the 20182020 annual meeting of stockholders will be ineligible for presentation at the meeting unless the stockholder gives timely notice of the proposal in writing to our Secretary at our principal executive offices and otherwise complies with the provisions of our Bylaws. To be timely, our Bylaws provide that we must have received the stockholder’s notice not lessno later than 90 daysMarch 21, 2020, and not moreno earlier than 120 days in advance of the first anniversary of the date this Proxy Statement was released to our stockholders in connection with the Annual Meeting.February 20, 2020. However, if the date of the 20182020 annual meeting of stockholders is more than 30 days before or more than 60 days after the first anniversary of the Annual Meeting, we must receive the stockholder’s notice not earlier than the close of business on the 120th day before the 20182020 annual meeting of stockholders and not later than the close of business on the later of (1) the 90th day before the 20182020 annual meeting of stockholders and (2) the 10th day following the day on which public announcement of the date of the 20182020 annual meeting of stockholders is first made. All stockholder proposals for our 20182020 annual meeting of stockholders should be sent to our Secretary, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142. Other Stockholder Communications Generally, stockholders who have questions or concerns should contact our Investor Relations department at (781)(781) 464-2442. However, stockholders who wish to communicate directly with our Board of Directors, or any individual director, should direct questions in writing to our Secretary, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142. Communications addressed in this manner will be forwarded directly to our Board of Directors or named individual director(s). Incorporation by Reference Notwithstanding anything to the contrary set forth in any of our previous filings under the securities laws that might incorporate future filings, including this Proxy Statement, in whole or in part, the Compensation Committee Report, the Audit Committee Report, the content ofwww.biogen.com, including the charters of the committees of our Board of Directors, Corporate Governance Principles, Related Person Transaction Policy, Conflicts of Interest Policy, and Code of Business Conduct and Bylaws, included or referenced in this Proxy Statement shall not be incorporated by reference into any such filings. Copies of Annual Meeting Materials Some banks, brokers and other nominee record holders may be participating in the practice of householding proxy statements and annual reports. This means that, unless you have instructed otherwise, only one copy of this Proxy Statement, annual report,Annual Report or Notice of Internet Availability of Proxy Materials, as applicable, may have been sent to multiple stockholders in your household.We will promptly deliver a separate copy of any of these documents without charge to you if you write or call Investor Relations, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142,(781) 464-2442.464-2442.If you want to receive separate copies of our proxy statement, annual report or Notice of Internet Availability of Proxy Materials, as applicable, in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker or other nominee record holder, or you may contact us at the above address or phone number. Manner and Cost of Proxy Solicitation Biogen pays the cost of soliciting proxies. In addition to solicitation by mail, our directors, officers and employees may contact you in person, by telephone or by email or other electronic means. None of our directors, officers or employees will receive additional compensation for soliciting you. We will reimburse brokerage houses, banks, custodians and other nominees and fiduciaries forout-of-pocket expenses incurred in forwarding our proxy solicitation materials to, and obtaining instructions relating to such materials from, beneficial owners of our common stock. Georgeson LLC, New York, New York, has been retained to assist us in the solicitation of proxies at a fee estimated not to exceed $11,000. | | | | | | 66 | |  | |  |
APPENDIX A GAAP toNon-GAAP Reconciliation Diluted Earnings Per Share and Net Income Attributable to Biogen Inc. (Unaudited,unaudited, $ in millions, except per share amounts) | | | | | | | | For the Twelve Months Ended | | | | For the Twelve Months Ended | | | | | | December 31,
2016 | | | December 31,
2015 | | | December 31, 2018 | | December 31, 2017 | GAAP earnings per share – Diluted | | $ | 16.93 | | | $ | 15.34 | | | | $21.58 | | | | $11.92 | | Adjustments to GAAP net income attributable to Biogen Inc. (as detailed below) | | | 3.29 | | | | 1.67 | | | | 4.62 | | | | 9.89 | | Non-GAAP earnings per share – Diluted | | $ | 20.22 | | | $ | 17.01 | | | | $26.20 | | | | $21.81 | |
| | | | | | | | For the Twelve Months Ended | | | | For the Twelve Months Ended | | | | | | December 31,
2016 | | | December 31,
2015 | | | December 31, 2018 | | December 31, 2017(1) | GAAP net income attributable to Biogen Inc. | | $ | 3,702.8 | | | $ | 3,547.0 | | | | $4,430.7 | | | | $2,539.1 | | Adjustments: | | | | | | TECFIDERA litigation settlement and license chargesA | | | 454.8 | | | | — | | | Amortization of acquired intangible assets | | | 373.6 | | | | 365.3 | | | (Gain) loss on fair value remeasurement of contingent consideration | | | 14.8 | | | | 30.5 | | | (Gain) loss on deconsolidation of variable interest entities | | | (4.4) | | | | — | | | Amortization of acquired intangible assetsA, B | | | | 747.3 | | | | 814.7 | | Acquiredin-process research and development | | | | 112.5 | | | | 120.0 | | Research and developmentC | | | | 10.0 | | | | — | | (Gain) loss on fair value remeasurement of contingent considerationD | | | | (12.3) | | | | 62.7 | | Premium paid on purchase of Ionis common stockE | | | | 162.1 | | | | — | | (Gain) loss on equity security investments | | | | (128.0) | | | | — | | Net distribution to noncontrolling interestsF | | | | 43.7 | | | | 132.4 | | Restructuring, business transformation and other cost saving initiatives: | | | 2017 corporate strategy implementationG | | | | 10.9 | | | | 18.5 | | Restructuring chargesG | | | | 12.0 | | | | 0.9 | | Hemophilia business separation costs | | | 18.1 | | | | — | | | | — | | | | 19.2 | | Restructuring, business transformation and other cost saving initiatives: | | | | | | Restructuring chargesB | | | 33.1 | | | | 93.4 | | | Cambridge manufacturing facility rationalization costsC | | | 54.8 | | | | — | | | Income tax effect related to reconciling items | | | (224.9) | | | | (104.3) | | | | (146.6) | | | | (235.7) | | Elimination of deferred tax assetH | | | | 10.6 | | | | — | | Tax reformI | | | | 124.9 | | | | 1,173.6 | | Non-GAAP net income attributable to Biogen Inc. | | $ | 4,422.7 | | | $ | 3,931.9 | | | | $5,377.8 | | | | $4,645.4 | |
Free Cash Flow Reconciliation (unaudited, $ in millions) (A) | | | | | | | | | | For the Twelve
Months Ended | | | December 31, 2018 | Net cash flows provided by operating activities | | | | $ 6,187.7 | | Purchases of property, plant and equipment (Capital Expenditures) | | | | (770.6) | | Contingent consideration related to Fumapharm AG acquisition | | | | (1,500.0) | | Free Cash Flow | | | | $ 3,917.1 | |
| (1) | On February 1, 2017, we completed thespin-off of our hemophilia business. Our consolidated results of operations reflect the financial results of our hemophilia business through January 31, 2017. |
AUnder our | In January 2017 we entered into a settlement and license agreement withamong Biogen Swiss Manufacturing GmbH, Biogen International Holding Ltd., Forward Pharma A/S (Forward Pharma), and certain related parties, which was effective February 1, 2017. Pursuant to this |
| | | | | | A-1 | |  | |  |
Appendix A(continued) | agreement, we obtained U.S. and rest of world licenses to Forward Pharma’s intellectual property, including Forward Pharma’s intellectual property related to TECFIDERA. In exchange, we paid Forward Pharma $1.25 billion in cash. The $455cash, of which $795.2 millionpre-tax charge was recognized duringas an intangible asset in the twelve months ended December 31, 2016 representsfirst quarter of 2017. |
| We have two intellectual property disputes with Forward Pharma, one in the portionU.S. and one in the European Union, concerning intellectual property related to TECFIDERA. |
| In March 2017 the U.S. intellectual property dispute was decided in our favor. Forward Pharma appealed to the U.S. Court of Appeals for the Federal Circuit. We evaluated the recoverability of the $1.25 billion cash payment that is attributableU.S. asset acquired from Forward Pharma and recorded a $328.2 million impairment charge in the first quarter of 2017 to adjust the carrying value of the acquired U.S. asset to fair value reflecting the impact of the developments in the U.S. legal dispute and continued to amortize the remaining net book value of the U.S. intangible asset in our salesconsolidated statements of TECFIDERA duringincome utilizing an economic consumption model. The U.S. Court of Appeals for the period April 2014 through December 31, 2016.Federal Circuit upheld the U.S. Patent and Trademark Office’s March 2017 ruling and in January 2019 denied Forward Pharma’s petition for rehearing. We evaluated the recoverability of the U.S. asset based upon these most recent developments and recorded a $176.8 million impairment charge in the fourth quarter of 2018 to reduce the remaining net book value of the U.S. asset to zero. |
(B) | Restructuring chargesIn March 2018 the European Patent Office (EPO) revoked Forward Pharma’s European Patent No. 2 801 355. Forward Pharma has filed an appeal to the Technical Boards of Appeal of the EPO and the appeal is pending. Based upon our assessment of this ruling, we continue to amortize the remaining net book value of the rest of world intangible asset in our consolidated statements of income utilizing an economic consumption model.
|
| Amortization of acquired intangible assets for the twelve months ended December 31, 20162017, also includes a $31.2 millionpre-tax impairment charge related to our acquired and 2015 include $8.0 millionin-licensed rights and $93.4 million, respectively,patents intangible asset associated with ZINBRYTA after the initiation of costs incurred in connection with our 2015 corporate restructuring. Restructuring chargesan European Medicines Agency review (referred to as an Article 20 Procedure) of ZINBRYTA following the report of a case of fatal fulminant liver failure, as well as four cases of serious liver injury. |
| B | Amortization of acquired intangible assets for the twelve months ended December 31, 2016 include2018, includes the impact of impairment charges totaling $189.3 million related to certainin-process research and development (IPR&D) assets associated with our vixotrigine (BIIB074) program. |
| During the third quarter of $17.72018 we completed a Phase 2b study of vixotrigine for the treatment of painful lumbosacral radiculopathy (PLSR). The study did not meet its primary or secondary efficacy endpoints; therefore, we discontinued development of vixotrigine for the treatment of PLSR and we recognized an impairment charge of approximately $60.0 million incurred in connection with additional cost savings measures primarily intendedduring the third quarter of 2018 to realign our organizational structure in anticipationreduce the fair value of the changes in rolesrelated IPR&D intangible asset to zero. In addition, we delayed the initiation of the Phase 3 studies of vixotrigine for the treatment of trigeminal neuralgia (TGN) as we awaited the outcome of ongoing interactions with the U.S. Food and workforce resultingDrug Administration (FDA) regarding the design of the Phase 3 studies, a more detailed review of the data from the Phase 2b study of vixotrigine for the treatment of PLSR and insights from the Phase 2 study of vixotrigine for the treatment of small fiber neuropathy. We reassessed the fair value of our decisionvixotrigine program for the treatment of TGN using reduced expected lifetime revenues, higher expected clinical development costs and a lower cumulative probability of success and, as a result of that assessment, we recognized an impairment charge of $129.3 million during the third quarter of 2018 to spin offreduce the fair value of the IPR&D intangible asset associated with our hemophilia business,vixotrigine program for the treatment of TGN to $41.8 million. |
| C | GAAP research and to achieve further targeted cost reductions. Restructuring chargesdevelopment expense for the twelve months ended December 31, 2016 also2018, include severance chargesa $10.0 million contingent consideration payment accrued in relation to the acquisition of $7.4 millionan asset. |
| D | During the third quarter of 2018, we adjusted the fair value of our contingent consideration obligations related to employee separation costsour vixotrigine program for the treatment of TGN to reflect the lower cumulative probabilities of success, which resulted in a gain of $89.6 million. |
| In late December 2018 we received feedback from the FDA regarding the design of the Phase 3 studies of vixotrigine for the treatment of TGN. Following this feedback, we are now planning to initiate the Phase 3 studies for our vixotrigine program for the treatment of TGN and, as a result, we adjusted the fair value of our decisioncontingent consideration obligations related to vacateour vixotrigine program for the treatment of TGN to reflect increased probabilities of success and cease manufacturingrecognized a loss of $80.6 million in Cambridge, MAthe fourth quarter of 2018. |
| E | In June 2018 we closed a newten-year exclusive agreement with Ionis Pharmaceuticals, Inc. (Ionis) to develop novel antisense oligonucleotide drug candidates for a broad range of neurological diseases for a total payment of $1.0 billion consisting of an upfront payment of $375.0 million and vacatethe purchase of approximately 11.5 million shares of Ionis’ common stock at a cost of $625.0 million. |
| The 11.5 million shares of Ionis’ common stock were purchased at a premium to their fair value at the transaction closing date. The premium consisted of acquiring the shares at a price above the fair value based on the trailing10-day weighted-average close price prior to entering into the agreement in April 2018 and the effect of certain holding period restrictions. We recorded an asset of $462.9 million in investments and other assets in our warehouseconsolidated balance sheets reflecting the fair value of the common stock as of the purchase date and a charge of $162.1 million to research and development expense in Somerville, MA.our consolidated statements of income during the second quarter of 2018 reflecting the premium paid for the common stock. |
| | | | | | A-2 | |  | |  |
Appendix A(continued) (C)F | Cambridge manufacturing facility rationalization costs reflectIn October 2017 we amended the terms of our collaboration and license agreement with Neurimmune SubOne AG (Neurimmune). Under the amended agreement, we made a $150.0 million payment to Neurimmune in exchange for a 15% reduction in the previously negotiated royalty rates payable on products developed under this agreement. In May 2018 we made an additional depreciation,$50.0 million payment to Neurimmune to further reduce the write-down of excess inventory and other direct costs associated with our decisionpreviously negotiated royalty rates payable on products developed under this agreement by an additional 5%.
|
| Net distribution to vacate and cease manufacturing in Cambridge, MA and vacate our warehouse in Somerville, MA. Additional depreciation expense, which totaled $45.5 millionnoncontrolling interest for the twelve months ended December 31, 2016, is included2018, reflects the $50.0 million payment made to Neurimmune, net of Neurimmune’s tax, in cost of sales, excluding amortization of acquired intangible assets in our condensed consolidated statements of income. Also reflected in this amountMay 2018. |
| Net distribution to noncontrolling interest for the twelve months ended December 31, 20162017, reflects the $150.0 million payment made to Neurimmune, net of Neurimmune’s tax, in October 2017. |
| G | 2017 corporate strategy implementation and restructuring charges are chargesrelated to our efforts to create a leaner and simpler operating model. |
| H | Elimination of $6.9deferred tax asset due to Samsung Bioepis Co., Ltd. qualifying as a corporate joint venture for accounting purposes. |
| I | The Tax Cuts and Jobs Act of 2017 (2017 Tax Act) resulted in significant changes to the U.S. corporate tax system. These changes include a federal statutory rate reduction from 35% to 21%, the elimination or reduction of certain domestic deductions and credits and limitations on the deductibility of interest expense and executive compensation. The 2017 Tax Act also transitions international taxation from a worldwide system to a modified territorial system and includes base erosion prevention measures onnon-U.S. earnings, which has the effect of subjecting certain earnings of our foreign subsidiaries to U.S. taxation as globallow-taxed income (GILTI). During the fourth quarter of 2018 we elected to recognize deferred taxes for basis differences expected to reverse as GILTI is incurred and have established initial deferred tax balances, as of the enactment date of the 2017 Tax Act. |
| During the fourth quarter of 2017 we recognized within our provision for income taxes a $1.2 billion provisional estimate pursuant to U.S. Securities and Exchange Commission Staff Accounting Bulletin No. 118. Our provisional estimate included an amount of $989.6 million associated with aone-time mandatory deemed repatriation tax on accumulated foreign subsidiaries’ previously untaxed foreign earnings (the Transition Toll Tax) and $184.0 million related to the impact of remeasuring our deferred tax balances to reflect the new federal statutory rate and other changes to U.S. tax law. |
| Tax reform amounts for the twelve months ended December 31, 2018, reflect the effect of an expense of $135.8 million related to the establishment of GILTI deferred taxes. |
| Tax reform amounts for the twelve months ended December 31, 2018, also reflect the effect of a net reduction of $34.6 million to our 2017 preliminary Transition Toll Tax estimate, an expense of $12.7 million for the write-downremeasurement of excess inventory, which are includedour deferred tax balance and an $11.0 million expense to reflect other aspects of the 2017 Tax Act. |
| The final determination of the Transition Toll Tax and remeasurement of our deferred assets and liabilities was completed in costthe fourth quarter of sales, excluding amortization of acquired intangible assets in our condensed consolidated statements of income.2018. |
Use ofNon-GAAP Financial Measures We supplement our consolidated financial statements presented on a GAAP basis by providing additional measures which may be considered“Non-GAAP” financial measures under applicable SEC rules. We believe that the disclosure of theseNon-GAAP financial measures provides additional insight into the ongoing economics of our business and reflects how we manage our business internally, set operational goals and formsform the basis of our management incentive programs. These | | | | | A-1 | |  | |  |
Appendix A(continued)
Non-GAAP financial measures are not in accordance with generally accepted accounting principles in the United States and should not be viewed in isolation or as a substitute for reported, or GAAP, net income attributable to Biogen Inc. and, diluted earnings per share.share and net cash flows provided by operating activities. Our“Non-GAAP net income attributable to Biogen Inc.” and“Non-GAAP earnings per share – Diluted” financial measures exclude the following items from “GAAP net income attributable to Biogen Inc.” and “GAAP earnings per share – Diluted”: 1. Purchase accounting, merger-related and merger-related adjustments.other adjustments We exclude certain purchase accounting related items associated with the acquisition of businesses, assets and amounts in relation to the consolidation or deconsolidation of variable interest entities for which we are the primary beneficiary. These adjustments include, but are not limited to, charges forin-process research IPR&D and development,certain milestones, the amortization of certain acquired intangible assets and charges or credits from the fair value remeasurement of our contingent consideration obligations. | | | | | | A-3 | |  | |  |
Appendix A(continued) 2. Hemophilia business separation costs.costs We have excluded costs that are directly associated with the set up and spin offspin-off of our hemophilia business into an independent, publicly-traded company.on February 1, 2017. These costs represent incremental third partythird-party costs attributable solely to hemophilia separationspin-off and set up activities. 3. Restructuring, business transformation and other cost saving initiatives.initiatives We exclude costs associated with the company’sour execution of certain strategies and initiatives to streamline operations, achieve targeted cost reductions, rationalize manufacturing facilities or refocus R&D activities. These costs may include employee separation costs, retention bonuses, facility closing and exit costs, asset impairment charges or additional depreciation when the expected useful life of certain assets have been shortened due to changes in anticipated usage and other costs or credits that management believes do not have a direct correlation to ouron-going ongoing or future business operations. 4. Other items.(Gain) loss on equity security investments Effective January 2018 we exclude unrealized and realized gains and losses and discounts or premiums on our equity security investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations. 5. Other items We evaluate other items of income and expense on an individual basis and consider both the quantitative and qualitative aspects of the item, including (i) its size and nature, (ii) whether or not it relates to our ongoing business operations and (iii) whether or not we expect it to occur as part of our normal business on a regular basis, including in the fourth quarter of 2016, TECFIDERA litigation settlement and license charges.basis. We also include an adjustment to reflect the related tax effect of all reconciling items within our reconciliation of our GAAP toNon-GAAP net income attributable to Biogen Inc. and diluted earnings per share. “Free Cash Flow” is defined as net cash flows provided by operating activities less purchases of property, plant and equipment and contingent consideration related to our acquisition of Fumapharm AG as disclosed in our 2018 Annual Report on Form10-K. | | | | | A-2A-4 | |  | |  |
APPENDIX B
Biogen Inc. 2017 Omnibus Equity Plan
BIOGEN INC.
2017 OMNIBUS EQUITY PLAN
1. Defined Terms
Exhibit A, which is incorporated by reference, defines certain capitalized terms used in the Plan and sets forth certain operational rules related to those terms.
2. Purpose; Term
The Plan provides for the grant of Awards consisting of or based on the Stock of the Company. The purpose of the Plan is to attract and retain employees of the Company and its Affiliates, to provide an incentive for them to generate stockholder value by contributing to the appreciation of the Company’s Stock price and to enable them to participate in the growth of the Company by granting Awards with respect to the Company’s Stock. No Awards may be granted under the Plan more than ten (10) years after the Effective Date, but Awards granted prior to that date may continue in accordance with their terms.
3. Administration
The Plan shall be administered by the Committee. The Board may in any instance perform any of the functions of the Committee hereunder and the Committee may delegate such of its duties, powers and responsibilities as it may determine in accordance with applicable legal requirements, including Section 157(c) of the Delaware General Corporation Law (references herein to the Committee shall include the Board or the person or persons so delegated to the extent of such delegation, as applicable).
The Committee shall select the Participants to receive Awards and shall determine the terms and conditions of the Awards. The Committee has discretionary authority, subject only to the express provisions of the Plan, to interpret the Plan; determine eligibility for and grant Awards; determine, modify or waive the terms and conditions of any Award; determine the form of settlement of Awards (whether in cash, shares of Stock or other property); prescribe forms, rules and procedures and otherwise do all things necessary or desirable to carry out the purposes of the Plan. Determinations of the Committee made under the Plan will be conclusive and will bind all parties.
4. Eligibility
All employees of the Company (or of any Affiliate) are eligible to be Participants in the Plan.
5. Stock Available for Awards
A.Shares Available. Subject to the other subsections of this Section 5 and subject to adjustment as provided in Section 10, no more than 8,000,000 shares of Stock in the aggregate may be delivered under or in satisfaction of Awards, plus the number of shares of Stock that, as of the date of adoption of the Plan, either: (i) remain available for grant under the 2008 Plan (including shares available under such plan by reason of a predecessor plan) or (ii) are subject to awards under 2008 Plan and on or after the date of adoption are cancelled, surrendered, exchanged, terminated or forfeited for any reason whatsoever in accordance with the terms of such plan. Shares issued under the Plan may consist of authorized but unissued shares or treasury shares. No fractional shares will be issued under the Plan.
B.Fungible Share Plan. Each share of Stock subject to an Award consisting of Options and/or SARs shall be counted against the limits set forth in Section 5.A as one (1) share. Each share of Stock subject to any Award other than an Award consisting of Options and/or SARs shall be counted against the limits set forth in Section 5.A as one andone-half (1.5) shares.
C.Reversion to the Plan. For the avoidance of doubt, if (i) an outstanding Award for any reason expires or is terminated or canceled without having been exercised or settled in full, (ii) if shares of Stock acquired pursuant to an Award subject to
| | | | | B-1 | |  | |  |
Appendix B(continued)
forfeiture or repurchase are forfeited or repurchased by the Company or (iii) on or after the date of adoption of the Plan, an outstanding award under the 2008 Plan is cancelled, surrendered, exchanged, terminated or forfeited in accordance with the terms of the 2008 Plan, the shares of Stock allocable to such expired, terminated, cancelled, exchanged or forfeited, as applicable, portion of such Award or such forfeited or repurchased shares of Stock, shall again be available for delivery under the Plan in an amount determined in accordance with Section 5.B. Shares of Stock shall not be deemed to have been delivered in satisfaction of Awards under the Plan to the extent that any portion of an Award is settled in cash or other property (other than shares of Stock). Upon delivery of shares of Stock in settlement of a SAR, the full number of shares of Stock covered by such SAR shall be treated as delivered under the Plan (and not only the number of shares of Stock delivered in settlement of such Award). Shares of Stock withheld from an Award in satisfaction of withholding taxes as described in Section 9.I or in payment of the exercise price or purchase price of any Award shall not again be available for delivery under the Plan.
D.Certain Other Company Awards. To the extent consistent with the requirements of Section 422 and the regulations thereunder, and other applicable legal requirements (including applicable stock exchange requirements), Stock issued under awards granted by another company (“other company awards”) and assumed by the Company in connection with a merger, consolidation, stock purchase or similar transaction, or issued by the Company under awards substituted for other company awards in connection with a merger, consolidation, stock purchase or similar transaction, shall not reduce the shares of Stock available for Awards under the Plan and such other company awards shall not be subject to the individual limits described in Section 5.E. Such awards may have terms inconsistent with the terms of the Plan.
E.Limit on Individual Grants. The maximum number of shares of Stock subject to Options, SARs, RSUs, RSAs and Other Awards that may be granted to any Participant in any calendar year is 1,500,000 shares of Stock in the aggregate, subject to adjustment as provided in Section 10. The foregoing limit refers to the maximum number of shares of Stock that may be delivered, or the value of which may be paid in cash or other property (based on the fair market value of the shares of Stock or such other property on the date of payment), under Awards granted during the calendar year. The foregoing limit will be construed in a manner consistent with Section 162(m), including, without limitation, where applicable, the rules under Section 162(m) pertaining to permissible deferrals of exempt awards.
6. Options
A.Grant of Options. Subject to the provisions of the Plan, the Committee may grant both (i) Options to purchase up to a maximum of 1,000,000 shares of Stock that are intended to comply with the requirements of Section 422 (“ISOs”) and (ii) Options that are not intended to comply with such requirements (“NQSOs”). Eligibility for ISOs is limited to employees of the Company or of a “parent corporation” or “subsidiary corporation” of the Company as those terms are defined in Section 424 of the Code. Each Option shall be clearly identified in the applicable Award agreement as either an ISO or an NQSO, but if no such identification is made, the Option shall be treated as an NQSO. The Committee shall determine the number of shares subject to each Option and the exercise price therefor, which shall not be less than 100% of the Fair Market Value of the Stock on the date of grant. An ISO granted to an employee described in Section 422(b)(6) of the Code must have an exercise price that is not less than 110% of such Fair Market Value.
B.Terms and Conditions. Each Option shall be exercisable at such times and subject to such terms and conditions as the Committee may specify in the Award agreement or thereafter. An ISO may not be exercisable after the period provided in Section1.422-2(d) of the Treasury Regulations. The Committee may impose such conditions with respect to the exercise of Options, including conditions relating to applicable federal or state securities laws, as it considers necessary or advisable. At the time of the grant of an Option, the Committee may impose such restrictions or conditions to the vesting of such Option as it, in its absolute discretion, deems appropriate, including requiring the achievement of Performance Criteria. The Expiration Date of each Option shall be ten (10) years from the date of grant thereof, or at such earlier time as the Committee shall state in the Award agreement.
C.Payment. No shares of Stock shall be delivered pursuant to any exercise of an Option until payment in full of the exercise price therefor is received by the Company. Such payment may be made in whole or in part in cash or, to the extent
| | | | | B-2 | |  | |  |
Appendix B(continued)
legally permissible and expressly permitted by the Committee at or after the grant of the Option, by delivery of other property such as shares of Stock (for which the Committee may require a holding period), valued at their Fair Market Value on the date of delivery or such other lawful consideration, including in accordance with a cashless exercise, as the Committee may determine or any combination of the foregoing permitted forms of payment.
7. SARs
A.Grant of SARs. Subject to the provisions of the Plan, the Committee may grant SARs. The Committee shall determine at the time of grant or thereafter whether SARs are settled in cash, Stock or other securities of the Company, Awards or other property, and may define the manner of determining the excess in value of the shares of Stock over the base value of the SAR. The Committee shall fix the base value of each SAR, which shall not be less than 100% of the Fair Market Value of the Stock on the date of grant.
B.Terms and Conditions. Each SAR shall be exercisable at such times and subject to such terms and conditions as the Committee may specify in the Award agreement or thereafter. The Committee may impose such conditions with respect to the exercise of SARs, including conditions relating to applicable federal or state securities laws, as it considers necessary or advisable. At the time of the grant of a SAR, the Committee may impose such restrictions or conditions to the vesting of such SAR as it, in its absolute discretion, deems appropriate, including requiring the achievement of Performance Criteria. The Expiration Date of each SAR shall be ten (10) years from the date of grant thereof, or at such earlier time as the Committee shall state in the Award agreement.
8. RSUs, RSAs and Other Awards
A.RSUs. Subject to the provisions of the Plan, the Committee may grant RSUs. Each RSU shall represent the unfunded and unsecured commitment of the Company to deliver to the Participant at a specified future date or dates one or more shares of Stock or, if specified in the Award, cash equal to the Fair Market Value of the Stock subject to the Award, in any case subject to the satisfaction of any vesting or other terms and conditions established with respect to the Award as the Committee may determine. The Committee may make Awards of RSUs that are subject to restrictions or forfeiture on such terms and conditions as the Committee may determine from time to time.
B.RSAs. Subject to the provisions of the Plan, the Committee may grant RSAs and determine the duration of the period (the “Restricted Period”) during which, and the conditions under which, the shares of Stock may be forfeited to the Company and the other terms and conditions of such Awards. RSAs may not be sold, assigned, transferred, pledged or otherwise encumbered during the Restricted Period. RSAs shall be evidenced in such manner as the Committee may determine. Any certificates issued in respect of RSAs shall be registered in the name of the Participant and, unless otherwise determined by the Committee, deposited by the Participant, together with a stock power endorsed in blank, with the Company. Upon the expiration of the Restricted Period, the Company shall deliver such shares of Stock, along with any certificates, to the Participant or if the Participant has died, to the Participant’s Designated Beneficiary.
C.Other Awards. Subject to the provisions of the Plan, the Committee may grant Awards (including Performance Awards) other than Options, SARs, RSUs or RSAs (“Other Awards”). Subject to the provisions of the Plan, the Committee shall have sole and complete authority to determine the persons to whom and the time or times at which such Other Awards shall be granted, the number of shares of Stock to be granted pursuant to such Other Awards, whether such Awards are to be settled in cash, Stock, other property or a combination of the foregoing, and all other conditions of such Other Awards.
D.Terms and Conditions. At the time of the grant of RSUs, RSAs or Other Awards, the Committee shall determine the price, if any, to be paid by the Participant for each share of Stock subject to the Award. At the time of the grant of RSUs, RSAs or Other Awards, as applicable, the Committee may impose such restrictions or conditions to the vesting of such Award as it, in its absolute discretion, deems appropriate, including requiring the achievement of Performance Criteria.
| | | | | B-3 | |  | |  |
Appendix B(continued)
9. General Provisions Applicable to Awards
A.Documentation and Legal Conditions on Delivery of Stock. Each Award shall be evidenced by a written or electronic document delivered or made available to the Participant or an agreement executed by the Participant specifying the terms and conditions thereof and containing such other terms and conditions not inconsistent with the provisions of the Plan as the Committee considers necessary or advisable to achieve the purposes of the Plan or to comply with applicable tax or other laws or accounting principles. The Company will not be obligated to deliver any shares of Stock pursuant to the Plan or to remove any restriction from shares of Stock previously delivered under the Plan until: (i) the Company’s counsel has approved all legal matters in connection with the issuance and delivery of such shares; (ii) if the outstanding Stock is at the time of delivery listed on any stock exchange or national market system, the shares to be delivered have been listed or authorized to be listed on such exchange or system upon official notice of issuance and (iii) all conditions of the Award have been satisfied or waived. If the sale of Stock has not been registered under the Securities Act of 1933, as amended, or if the Company determines that the registration statement covering the sale of Stock is not available, the Company may defer the sale until such time as it determines that the registration statement is available and may delay the applicability of any provisions of the Award during any period of unavailability. The Company may require, as a condition to the exercise of an Award or the delivery of shares of Stock under an Award, such representations or agreements as counsel for the Company may consider appropriate to avoid violation of the Securities Act of 1933, as amended, or any applicable state ornon-U.S. securities law. The Company may require that certificates evidencing Stock issued under the Plan bear an appropriate legend reflecting any restriction on transfer applicable to such Stock and the Company may hold the certificates pending lapse of the applicable restrictions.
B.Performance Criteria. The Committee may establish Performance Criteria on which the granting of Performance Awards, or the vesting of Performance Awards, will be subject. The Committee shall determine whether any Performance Criteria so established have been achieved, and if so to what extent, and its determination shall be binding on all persons.
C.Minimum Vesting Period. To the extent an Award is to vest based solely upon the continued employment of the Participant, such Award shall vest pursuant to a schedule that provides for vesting in three equal increments on each of the first three anniversaries of the date of grant, or such longer period as the Committee may determine; provided, however, that up to 500,000 shares of Stock may be made subject to Awards with a time-based vesting schedule that provides for vesting sooner than the default schedule set forth in this Section 9.C; and, provided, further, that Awards shall be subject to accelerated vesting as set forth in Section 9.G, Section 10 or in a Participant’s Award agreement.
D.Committee Discretion. Awards may be made alone or in combination with other Awards, including Awards of other types. The terms of Awards of the same type need not be identical, and the Committee need not treat Participants uniformly (subject to the requirements of applicable law). Except as otherwise expressly provided by the Plan or a particular Award agreement, any determination with respect to an Award may be made by the Committee at the time of grant or at any time thereafter.
E.Dividends and Cash Payments. In the discretion of the Committee, any Award under the Plan may provide the Participant with (i) dividends or dividend equivalents payable (in cash, property or other Awards under the Plan) currently or deferred with or without interest and (ii) cash payments in lieu of or in addition to an Award. Any entitlement to such dividends, dividend equivalents, cash payments or similar entitlements will be established and administered either consistent with an exemption from, or in compliance with, the requirements of Section 409A and shall subject to such limits or restrictions as the Committee may impose.
F.Leaves of Absence. Awards held by a Participant on an approved leave of absence shall continue to vest in accordance with their terms during the leave of absence as if the Participant was an active employee unless otherwise agreed to in writing between the Company and the Participant or otherwise set forth in the Award agreement; provided, however, in the event of an ISO, such leave of absence shall not exceed ninety (90) days unless reemployment is guaranteed by law or contract.
| | | | | B-4 | |  | |  |
Appendix B(continued)
G.Termination of Employment. Unless the Committee expressly provides otherwise in an Award agreement, the following rules shall apply in connection with the termination of a Participant’s employment with the Company and its Affiliates. Immediately upon the termination of the Participant’s employment with the Company and its Affiliates, each Award requiring exercise will cease to be exercisable and each Award to the extent not then vested will be forfeited, except that:
(1) In the event of a termination of the Participant’s employment by reason of death or as a result of Disability, each Award held by a Participant immediately prior to his or her death or termination of employment as a result of Disability shall, to the extent not vested previously, become fully vested, and each Option, SAR and other Award requiring exercise, to the extent then exercisable, will remain exercisable by the Participant or the Participant’s executor or administrator or the person or persons to whom the Option or SAR is transferred by will or the applicable laws of descent and distribution, in each case for the lesser of: (i) theone-year period ending with the first anniversary of the Participant’s death or Disability, as applicable, or (ii) the period ending on the latest date on which such Option or SAR could have been exercised without regard to this subsection G, and shall thereupon terminate;
(2) In the event of the Participant’s Retirement, each Award, to the extent not vested previously, shall become vested as to 50% of the number of shares covered by such unvested Award and for an additional 10% of the number of shares covered by such unvested Award for every full year of consecutive employment by the Company or any of its Affiliates beyond ten (10) years, up to the remaining amount of the unvested Award; provided, however, that: (i) the applicable grants with respect to such Awards shall provide for payment terms that comply with, or are exempt from, the requirements of Section 409A and (ii) Awards subject to Performance Criteria intended to comply with Section 162(m) will vest according to the schedule contemplated in this Section 9.G(2) only to the extent consistent with the requirements of Section 162(m). In the event of the Participant’s Retirement, each Option, SAR and other Award requiring exercise, to the extent then exercisable (after giving effect to the accelerated vesting provided for herein), will remain exercisable for the lesser of: (a) the three-year period ending with the third anniversary of the Participant’s Retirement or (b) the period ending on the latest date on which such Option or SAR could have been exercised without regard to this subsection G, and shall thereupon terminate;
(3) In the event of the Participant’s termination of employment For Cause, each Award held by a Participant or a Participant’s permitted transferees, if any, immediately prior to such termination of employment (including any portion of the Award that is then exercisable) shall terminate at the commencement of business on the date of such termination; and
(4) In the event of the Participant’s termination of employment for any reason other than death, Disability, Retirement or For Cause, each Option, SAR and other Award requiring exercise held by a Participant immediately prior to such termination of employment, to the extent then exercisable, will remain exercisable for the lesser of: (i) the period ending six (6) months from the Participant’s termination date or (ii) the period ending on the latest date on which such Option or SAR could have been exercised without regard to this subsection G, and shall thereupon terminate; and each other Award shall terminate at the close of business on the date of such termination.
Subject to Section 9.O, unless the Committee expressly provides otherwise, a Participant’s employment with the Company and its Affiliates will be deemed to have ceased upon termination of the Participant’s employment with the Company and its Affiliates (whether or not the Participant continues in the service of the Company or its Affiliates in some capacity other than that of an employee of the Company or its Affiliates).
H.Transferability.No Award may be transferred other than by will or the laws of descent and distribution and may be exercised during the life of a Participant only by the Participant, except that, as to Options other than ISOs, the Committee may in its sole discretion permit certain transfers to the Participant’s family members or to certain entities controlled by the Participant or his or her family members.
I.Withholding Taxes. The Participant shall pay to the Company, or make provision satisfactory to the Committee for payment of, any taxes or social insurance contributions required by law to be withheld with respect to Awards under the Plan no later than the date of the event creating the tax liability. The Company and its Affiliates will, to the extent permitted by law, deduct any such tax or social insurance contributions from any payment of any kind due to the Participant hereunder or
| | | | | B-5 | |  | |  |
Appendix B(continued)
otherwise. In the Committee’s discretion, tax and social insurance contributions required by law to be withheld in respect of Awards may be paid in whole or in part in shares of Stock, including shares retained by the Company from the Award creating the obligation, valued at their Fair Market Value on the date of retention or delivery, but not in excess of the maximum withholding amount consistent with the Award being subject to equity accounting treatment under applicable accounting rules (including FASB ASC Topic 718 (or any successor provision)).
J.Option or SAR Repricing.Except in connection with a corporate transaction involving the Company (which term includes, without limitation, any stock dividend, stock split, extraordinary cash dividend, recapitalization, reorganization, merger, consolidation,split-up,spin-off, combination or exchange of shares) or as otherwise contemplated by Section 10 below, the Company may not, without obtaining stockholder approval, (i) amend the terms of outstanding Options or SARs to reduce the exercise price or base value of such Options or SARs, (ii) cancel outstanding Options or SARs in exchange for Options or SARs with an exercise price or base value that is less than the exercise price or base value of the original Options or SARs or (iii) cancel outstanding Options or SARs that have an exercise price or base value greater than the Fair Market Value of a share of Stock on the date of such cancellation in exchange for cash or other consideration.
K.Amendment of Award. Except as otherwise expressly provided in the Plan, the Committee may amend, modify or terminate any outstanding Award, including substituting therefor another Award of the same or a different type, changing the date of exercise or realization and converting an ISO to an NQSO; provided, however, that if stockholder approval is required by law or the rules of the applicable stock exchange on which the Stock of the Company is then publicly-traded, such amendment shall not become effective until such stockholder approval is obtained. Any such action shall require the Participant’s consent unless the Committee determines that the action would not materially and adversely affect the Participant.
L.Recovery of Compensation. The Committee may cancel, rescind, withhold or otherwise limit or restrict any Award at any time if the Participant is not in compliance with all applicable provisions of the Award agreement and the Plan or if the Participant engages in any Detrimental Activity. In addition, the Committee may provide that Awards and the proceeds from Awards or Stock acquired thereunder will be subject to forfeiture and disgorgement to the Company to the extent required or permitted by applicable Company policy, law or stock exchange listing standards. Each Participant, by accepting or being deemed to have accepted an Award under the Plan, agrees to cooperate fully with the Committee to effectuate any forfeiture or disgorgement required hereunder. The Participant (and neither the Committee nor the Company) will be solely responsible for any adverse tax or other consequences to a Participant that may arise in connection with this Section 9.L.
M.Foreign Nationals.The Committee may take any action consistent with the terms of the Plan, either before or after an Award has been granted, which the Committee deems necessary or advisable to comply with government laws or regulatory requirements of any foreign jurisdiction, including but not limited to modifying or amending the terms and conditions governing any Awards, establishingsub-plans under the Plan or adopting such procedures as the Committee may determine to be appropriate in response to differences in laws, rules, regulations or customs of such foreign jurisdictions with respect to tax, securities, currency, employment, accounting or other matters.
N.Deemed Exercise of Awards.On the Expiration Date on which a vested Award (or portion thereof) requiring exercise is scheduled to terminate in accordance with the Plan and the terms of the Award, if the per share exercise price or base value, as the case may be, of the Award is less than the Fair Market Value of a share of Stock on that date, the vested portion of the Award will be deemed to have been exercised at the close of business on that date. As promptly as practicable thereafter, the Company will deliver to the Participant the shares of Stock subject to the vested portion of the Award less that number of shares with a value that is equal to the aggregate Fair Market Value of: (i) the aggregate exercise price or base value, as the case may be, of the vested portion of the Award and (ii) the amount withheld, as determined by the Committee in accordance with Section 9.I, in satisfaction of any federal, state and local withholding of taxes or social insurance contributions related to the exercise.
| | | | | B-6 | |  | |  |
Appendix B(continued)
O.Section 409A. Notwithstanding any other provision of the Plan or any Award agreement to the contrary:
(1) Awards under the Plan are intended either to be exempt from the rules of Section 409A or to satisfy those rules, and shall be construed accordingly.
(2) To the extent required in order to avoid accelerated taxation and/or tax penalties under Section 409A, a Participant shall not be considered to have terminated employment with the Company and its Affiliates until the Participant would be considered to have incurred a “separation from service” from the Company and its Affiliates within the meaning of Section 409A (after giving effect to the presumptions contained therein).
(3) If a Participant is deemed on the date of the Participant’s termination of Employment to be a “specified employee” within the meaning of that term under Section 409A(a)(2)(B) of the Code, then, with regard to any payment that is considered nonqualified deferred compensation under Section 409A, to the extent applicable, payable on account of a “separation from service”, such payment will be made or provided on the date that is the earlier of (i) the expiration of thesix-month period measured from the date of such “separation from service” and (ii) the date of the Participant’s death (the “Delay Period”). Upon the expiration of the Delay Period, all payments delayed pursuant to this Section 9.O(3) (whether they would have otherwise been payable in a single lump sum or in installments in the absence of such delay) will be paid on the first business day following the expiration of the Delay Period in a lump sum and any remaining payments due under the Award will be paid in accordance with the normal payment dates specified for them in the applicable Award agreement.
(4) For purposes of Section 409A, each payment made under the Plan shall be treated as a separate payment.
P.Section 162(m). In the case of any Performance Award (other than an Option or SAR) intended to qualify for the performance-based compensation exception under Section 162(m), the Committee shall establish the Performance Criterion (or Criteria) applicable to the Award within the time period required under Section 162(m) and the grant, vesting or payment, as the case may be, of the Award will be conditioned upon the satisfaction of the Performance Criterion (or Criteria) as certified by the Committee. The Committee may, subject to the terms of the Plan, amend a previously granted Performance Award or take any other action that disqualifies such Award from the performance-based compensation exception under Section 162(m).
10. Effect of Certain Transactions
A.Covered Transactions
Except as otherwise expressly provided in an Award agreement:
(1) If the Covered Transaction is one in which there is an acquiring or surviving entity other than the Company or its Affiliate, the Committee shall provide for the assumption of some or all outstanding Awards or for the grant of new Awards in substitution therefor or the continuation of some or all of the Awards by the acquiror or survivor or an affiliate of the acquiror or survivor, except to the extent that the Committee pays out the Award pursuant to the provisions of Section 10.A(2).
(2) If the Covered Transaction is one in which holders of Stock will receive upon consummation a payment (whether cash,non-cash or a combination of the foregoing), the Committee may provide for payment (acash-out), with respect to some or all Awards or any portion thereof (whether or not vested), equal in the case of each affected Award or portion thereof to the excess, if any, of (i) the Fair Market Value of one share of Stock times the number of shares of Stock subject to the Award or such portion, over (ii) the aggregate exercise or purchase price, if any, under the Award or such portion (in the case of a SAR, the aggregate base value above which appreciation is measured), in each case on such payment terms (which need not be the same as the terms of payment to holders of Stock) and other terms, and subject to such conditions, as the Committee determines; provided, that the Committee shall not exercise its discretion under this Section 10.A(2) with respect to an Award or portion thereof providing for nonqualified deferred compensation subject to Section 409A in a manner that would constitute an extension or acceleration of, or other change in, payment terms if such change would be inconsistent with the applicable requirements of Section 409A. For avoidance of doubt, in the event that the aggregate
| | | | | B-7 | |  | |  |
Appendix B(continued)
exercise or purchase price of the Award exceeds the aggregate Fair Market Value of the Stock subject to the Award, the Award will be deemed to be cashed out for a payment of zero.
(3) Each Award will terminate upon consummation of the Covered Transaction, other than Awards assumed, substituted or continued pursuant to Section 10.A(1). For avoidance of doubt, in the event that the Awards are not cashed out (or deemed cashed out) as provided in Section 10.A(2), such Awards shall be assumed, substituted or continued as provided in Section 10.A(1).
B.Corporate Transaction. Except as otherwise provided in the Award agreement, if at any time within two (2) years after the effective date of a Corporate Transaction there is an Involuntary Employment Action with respect to any Designated Employee, each then outstanding Award assumed, substituted or continued under Section 10.A(1) and held by such Designated Employee (or a permitted transferee of such person) shall, upon the occurrence of such Involuntary Employment Action, automatically accelerate so that each such Award shall become fully vested or exercisable, as applicable, immediately prior to such Involuntary Employment Action. Upon the occurrence of an Involuntary Employment Action with respect to a Designated Employee, any outstanding Options or SARs held by such Designated Employee (and a permitted transferee of such person) shall be exercisable for one (1) year following the Involuntary Employment Action or, if earlier, within the originally prescribed term of the Option or SAR.
C.Corporate Change in Control. Except as otherwise provided in the Award agreement, if at any time within two (2) years after the effective date of a Corporate Change in Control there is an Involuntary Employment Action with respect to any Designated Employee, each then outstanding Award assumed, substituted or continued under Section 10.A(1) and held by such Designated Employee (or a permitted transferee of such person) shall, upon the occurrence of such Involuntary Employment Action, automatically accelerate so that each such Award shall become fully vested or exercisable, as applicable, immediately prior to such Involuntary Employment Action. Upon the occurrence of an Involuntary Employment Action with respect to a Designated Employee, any outstanding Options or SARs held by such Designated Employee (and a permitted transferee of such person) shall be exercisable for one (1) year following the Involuntary Employment Action or, if earlier, within the originally prescribed term of the Option or SAR.
D.Changes In, Distributions With Respect To and Redemptions of the Stock.
(1) In the event of any stock dividend or other similar distribution of stock or other securities of the Company, stock split or combination of shares (including a reverse stock split), recapitalization, conversion, reorganization, consolidation,split-up,spin-off, combination, merger, exchange of stock, redemption or repurchase of all or part of the shares of any class of stock or any change in the capital structure of the Company or an Affiliate or other transaction or event that constitutes an equity restructuring within the meaning of FASB ASC Topic 718 (or any successor provision), the following shall be equitably adjusted (i) the number of shares that may be delivered as per Section 5, (ii) the individual limits described in Section 5.E, (iii) the number and kind of shares of stock or securities subject to Awards then outstanding or subsequently granted, (iv) exercise prices or base values, as the case may be, relating to outstanding Awards and (v) any other provision of Awards affected by such change.
(2) The Committee may also make equitable or proportionate adjustments of the type described in Section 10.D(1) to take into account distributions to stockholders other than stock dividends or normal cash dividends, material changes in accounting practices or principles, extraordinary dividends, mergers, consolidations, acquisitions, dispositions or similar transactions involving Stock or any other event other than those described in Section 10.D(1), if the Committee determines that adjustments are appropriate to avoid distortion in the operation of the Plan and to preserve the value and equity of Awards made hereunder, having due regard for: (i) the qualification of ISOs under Section 422; (ii) the continued exemption of the Awards from (or satisfaction by the Awards of the rules of) Section 409A, where applicable and (iii) in the case of Awards intended to qualify for the performance-based compensation exception Section 162(m), the continued qualification for that exception (unless otherwise determined by the Committee).
| | | | | B-8 | |  | |  |
Appendix B(continued)
(3) References in the Plan to shares of Stock will be construed to include any stock or securities resulting from an adjustment pursuant to this Section 10.
11. Miscellaneous
A.No Right to Employment. No person shall have any claim or right to be granted an Award. Neither the adoption, maintenance, nor operation of the Plan nor any Award hereunder shall constitute a contract of employment or confer upon any employee of the Company or of any Affiliate any right with respect to the continuance of his/her employment by or other service with the Company or any such Affiliate nor shall it or they be construed as affecting the rights of the Company (or Affiliate) to terminate the service of any person at any time or otherwise change the terms of such service, including, without limitation, the right to promote, demote or otherwisere-assign any employee from one position to another within the Company or any Affiliate.
B.No Rights as a Stockholder. Subject to the provisions of the applicable Award, no Participant or other person shall have any rights as a stockholder with respect to any shares of Stock to be issued under the Plan until he or she becomes the holder thereof. A Participant to whom an RSA is awarded shall be considered a stockholder of the Company at the time the Award is granted except as otherwise expressly provided in the applicable Award agreement.
C.Effective Date. The Plan became effective on the Effective Date.
D.Amendment of the Plan. The Committee may amend, suspend or terminate the Plan or any portion thereof at any time, subject to such stockholder approval as the Committee determines to be necessary or advisable. Further, under all circumstances, the Committee may, but shall not be required to, makenon-substantive administrative changes to the Plan in order to conform with or take advantage of governmental requirements, statutes or regulations. Except as provided in Section 9.L, no such amendment, modification or termination will adversely affect the rights of any Participant (without his or her consent) under any Award previously granted and no amendment will, without the approval of the stockholders of the Company, effectuate a change for which stockholder approval is required under applicable law or relevant stock exchange listing standards.
E.Governing Law. The provisions of the Plan shall be governed by and interpreted in accordance with the laws of the State of Delaware.
F.Limitation of Liability.Notwithstanding anything to the contrary in the Plan, neither the Company, nor any Affiliate, nor the Committee, nor any person acting on behalf of the Company, any Affiliate or the Committee, will be liable to any Participant, to any permitted transferee, to the estate or beneficiary of any Participant or any permitted transferee or to any other holder of an Award by reason of any acceleration of income, or any additional tax (including any interest and penalties), asserted by reason of the failure of an Award to satisfy the requirements of Section 422 or Section 409A or by reason of Section 4999 of the Code, or otherwise asserted with respect to the Award.
| | | | | B-9 | |  | |  |
Appendix B(continued)
EXHIBIT A
Definition of Terms
The following terms, when used in the Plan, will have the meanings and be subject to the provisions set forth below:
“2008 Plan” means the Company’s 2008 Amended and Restated Omnibus Equity Plan.
“Affiliate” means any corporation or other entity that stands in a relationship to the Company that would result in the Company and such corporation or other entity being treated as a single employer under Sections 414(b) or 414(c) of the Code, except that such Sections shall be applied by substituting “at least 50%” for “at least 80%” wherever applicable; provided, however, that in determining eligibility for the grant of an Option or SAR by reason of service for an Affiliate, “Affiliate” shall mean any corporation or other entity in a chain of corporations all of which have a controlling interest in another corporation or other entity in the chain, beginning with the parent entity and ending with the entity for which the Award recipient was providing services on the grant date of the Award (defining the term “controlling interest” based on “at least 50%” rather than “at least 80%”). The Company may at any time by amendment provide that different ownership thresholds apply (consistent with Section 409A, where applicable).
“Award” means any Option, SAR, RSA, RSU and any Other Award convertible into or otherwise based on Stock (including a Performance Award payable in cash), granted under the Plan.
“Board” means the Board of Directors of the Company.
“Code”means the Internal Revenue Code of 1986, as amended from time to time, or any successor law.
“Committee”means the Compensation and Management Development Committee of the Board.
“Company” means Biogen Inc., a Delaware corporation.
“Competitive Activity” shall include: (i) the rendering of services for any organization or engaging directly or indirectly in any business which is or becomes competitive with the Company, or which organization or business, or the rendering of services to such organization or business, is or becomes otherwise prejudicial to or in conflict with the interests of the Company; (ii) the disclosure to anyone outside the Company, or the use in other than the Company’s business, without prior written authorization from the Company, of any confidential information or material relating to the business of the Company, acquired by the Participant either during or after employment with the Company or (iii) any attempt directly or indirectly to induce any employee of the Company to be employed or perform services elsewhere or any attempt directly or indirectly to solicit the trade or business of any current or prospective customer, supplier or partner of the Company.
“Continuing Director” shall mean, as of any date of determination, any member of the Board who (i) was a member of the Board on March 24, 2017 or (ii) becomes a member of the Board subsequent to March 24, 2017 and was appointed, nominated for election or elected to the Board with the approval of a majority of the Continuing Directors who were members of the Board at the time of such appointment, nomination or election, provided that a director whose initial assumption of office is in connection with an actual or threatened election contest will not be considered a Continuing Director unless and until (a) such director has served on the Board for at least two (2) years and (b) the most recent reelection of such director has been approved by a majority of the Continuing Directors in office at the time of such approval.
“Corporate Change in Control” shall be deemed to have occurred upon the first of the following events:
(1) an event in which any Person, is or becomes the “beneficial owner” (as defined in Section 13(d) of the Exchange Act), together with all affiliates and associates (as such terms are used in Rule12b-2 under the Exchange Act) of such Person, directly or indirectly, of securities of the Company representing 50% or more of the combined voting power of the Company’s then outstanding securities;
(2) the consummation of the merger or consolidation of the Company with any other company, other than a merger or consolidation which would result in the voting securities of the Company outstanding immediately prior thereto continuing to
| | | | | B-10 | |  | |  |
Appendix B(continued)
represent (either by remaining outstanding or by being converted into voting securities of the surviving entity), more than 50% of the combined voting power of the voting securities of the Company or such surviving entity outstanding immediately after such merger; or
(3) at any time the Continuing Directors do not constitute a majority of the Board (or, if applicable, the board of directors of a successor to the Company).
Notwithstanding the foregoing, in any case where the occurrence of a Corporate Change in Control could affect the vesting of or payment under an Award subject to the requirements of Section 409A, to the extent required to comply with Section 409A, the term “Corporate Change in Control” shall mean an occurrence that both (i) satisfies the requirements set forth above in this definition and (ii) is a “change in control event” as that term is defined in the regulations under Section 409A.
“Corporate Transaction” means any of: (i) a consolidation, merger or similar transaction or series of related transactions, including a sale or other disposition of stock, in which the Company (or an Affiliate) is not the surviving corporation or which results in the acquisition of all or substantially all of the then outstanding Stock by a single person or entity or by a group of persons and/or entities acting in concert; (iii) a sale or transfer of all or substantially all of the Company’s assets or (iv) a dissolution or liquidation of the Company. Where a Corporate Transaction involves a tender offer that is reasonably expected to be followed by a merger described in clause (i) as determined by the Committee, the Corporate Transaction shall be deemed to have occurred upon consummation of the tender offer.
Notwithstanding the foregoing, in any case where the occurrence of a Corporate Transaction could affect the vesting of or payment under an Award subject to the requirements of Section 409A, to the extent required to comply with Section 409A, the term “Corporate Transaction” shall mean an occurrence that both (a) satisfies the requirements set forth above in this definition and (b) is a “change in control event” as that term is defined in the regulations under Section 409A.
“Covered Transaction” means a Corporate Change in Control or a Corporate Transaction.
“Delay Period” has the meaning set forth in Section 9.O(3).
“Designated Beneficiary”means the Participant’s estate.
“Designated Employee” means an employee designated by the Committee, in its sole discretion, as a “Designated Employee” for purposes of the Plan at any time prior to the effective date of a Corporate Transaction or Corporate Change in Control, as applicable.
“Detrimental Activity” shall include any action or failure to act that, in the sole determination of the Committee: (i)(a) constitutes financial malfeasance that is materially injurious to the Company, (b) violates the Company’s Code of Conduct, (c) results in the Company’s restatement of its earnings, financial results or financial statements or (d) results in a violation or breach of law or contract that is materially injurious to the Company or (ii) violates anynon-competition,non-disclosure ornon-solicitation agreement with the Company, or in the event that the Participant has not entered into any such agreement with the Company, the Participant engages in any Competitive Activity.
“Disability” shall exist for purposes of the Plan if the Company determines in its sole discretion that the Participant has been terminated as a result of the employee having become totally and permanently disabled. For this purpose, totally and permanently disabled means that the Participant is unable to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment that can be expected to result in death or can be expected to last for a continuous period of not less than 12 months.
“Effective Date”means the date the Plan was approved by the Company’s stockholders.
“Exchange Act” means the Securities Exchange Act of 1934, as amended from time to time, or any successor law.
“Expiration Date”means the latest date on which an Option, SAR or Other Award requiring exercise may be exercised pursuant to the Award agreement.
| | | | | B-11 | |  | |  |
Appendix B(continued)
“Fair Market Value” means, with respect to Stock, for so long as such Stock is readily tradable on an established securities market (within the meaning of Section 409A), the closing price on the day of the grant or measurement or, if the applicable date is not a trading day, on the most recent trading day immediately prior to the applicable date, and otherwise, the fair market value of such Stock determined by the Committee by a reasonable application of a reasonable valuation method (within the meaning of Section 409A).
“For Cause” shall be deemed to include, but is not limited to, dishonesty with respect to the Company or any Affiliate, insubordination, substantial malfeasance ornon-feasance of duty, unauthorized disclosure of confidential information, breach of, or a failure to comply with, the Company’s policies, procedures or codes of ethics or business conduct, breach by a Participant of any provision of any employment, nondisclosure,non-competition or similar agreement between the Participant and the Company or any Affiliate, the Participant’s commission of, or plea of nolo contendere to, a felony and other conduct that is, or could reasonably be expected to be, harmful or prejudicial to the business of the Company or an Affiliate. The determination of the Committee or its designee as to the existence of circumstances warranting a termination For Cause shall be conclusive. Notwithstanding the foregoing, in the event that the Participant is a party to an effective employment or similar agreement with the Company or an Affiliate which contains a “cause” definition, such definition shall be controlling for purposes of the Plan for so long as such agreement is in effect.
“Involuntary Employment Action” as to a Participant means the involuntary termination of a Participant’s employment with the Company following a Corporate Transaction or Corporate Change in Control, as applicable, (i) other than For Cause or (ii) upon the occurrence of any of the following circumstances: (a) any adverse and/or material alteration and diminution in the Participant’s authority, duties or responsibilities (other than a mere change in title or reporting relationship) as they existed immediately prior to the Corporate Transaction or Corporate Change in Control, as applicable, or as the same may be increased from time to time thereafter, (b) a reduction of the Participant’s base salary or a reduction in targeted bonus opportunity, in each case as in effect on the date prior to the Corporate Transaction or Corporate Change in Control, as applicable, or as the same may be increased from time to time thereafter or (c) relocation of the offices at which the Participant is employed which increases his or her daily commute by more than 100 miles on a round trip basis; provided, however, that in any case the Participant notifies the Chief Legal Officer or the Head of Human Resources of the Company in writing of the basis for his or her involuntary termination within ninety (90) days of the occurrence of the circumstances and the Company does not cure such circumstance within thirty (30) days thereafter.
“ISOs” has the meaning set forth in Section 6.A.
“NQSOs” has the meaning set forth in Section 6.A.
“Option” means the right to purchase shares of Stock of the Company for a specified period of time at a specified price.
“Other Award” has the meaning set forth in Section 8.C.
“other company award” has the meaning set forth in Section 5.D.
“Participant”means a person selected by the Committee to receive an Award under the Plan.
“Performance Award” means an Award subject to Performance Criteria. The Committee in its discretion may grant Performance Awards that are intended to qualify for the performance-based compensation exception under Section 162(m) and Performance Awards that are not intended to so qualify.
“Performance Criteria” means specified criteria, other than the mere continuation of employment or passage of time, the satisfaction of which is a condition for the grant, exercisability, vesting, payment or full enjoyment of an Award. For purposes of Performance Awards that are intended to qualify for the performance-based compensation exception under Section 162(m), a Performance Criterion shall be based on objectively determinable measures of performance relating to any of, or to any combination of, the following (measured either absolutely or comparatively (including, without limitation, by reference to an index or indices or the performance of one or more companies) and determined either on a consolidated basis or, as the context permits, on a divisional, functional, subsidiary, line of business, project or geographical basis or in
| | | | | B-12 | |  | |  |
Appendix B(continued)
combinations thereof and subject to such adjustments, if any, as the Committee specifies, consistent with the requirements of Section 162(m)): sales; revenues; assets; expenses; earnings before or after deduction for all or any portion of interest, taxes, depreciation, or amortization or other items, whether or not on a continuing operations or an aggregate or per share basis; return on equity, investment, capital or assets; one or more operating ratios; borrowing levels, leverage ratios or credit rating; market share; capital expenditures; cash flow; stock price; stockholder return; sales of particular products or services; customer acquisition, expansion or retention; acquisitions and divestitures (in whole or in part); joint ventures and strategic alliances; spin-offs,split-ups and the like; reorganizations; recapitalizations, restructurings, financings (issuance of debt or equity) or refinancings; or achievement of clinical trials or measurable research objectives. A Performance Criteria and any targets with respect thereto determined by the Committee shall be based on achievement of an objectively determinable performance goal. A Performance Criteria and any targets with respect thereto determined by the Committee need not be based upon an increase, a positive or improved result or avoidance of loss and may be based on GAAP,non-GAAP or other metrics as provided for herein. To the extent consistent with the requirements for satisfying the performance-based compensation exception under Section 162(m), the Committee may provide in the case of any Award intended to qualify for such exception that one or more of the Performance Criteria applicable to such Award will be adjusted in an objectively determinable manner to reflect events (for example, but without limitation, acquisitions or dispositions) occurring during the performance period that affect the applicable Performance Criterion or Criteria.
“Person” shall have the meaning given in Section 3(a)(9) of the Exchange Act, as modified and used in Sections 13(d) and 14(d) thereof, except that such term shall not include: (i) the Company or any of its Affiliates; (ii) a trustee or other fiduciary holding securities under an employee benefits plan of the Company or any of its Affiliates; (iii) an underwriter temporarily holding securities pursuant to an offering of such securities or (iv) a corporation or other business entity owned, directly or indirectly, by the stockholders of the Company in substantially the same proportions as their ownership of stock of the Company.
“Plan”means the Biogen 2017 Omnibus Equity Plan, as from time to time amended and in effect.
“Restricted Period” has the meaning set forth in Section 8.B.
“Retirement” as to any employee of the Company or any of its Affiliates shall mean such person’s leaving the employment of the Company and its Affiliates after reaching age 55 with ten (10) consecutive years of service with the Company or its Affiliates, but not including pursuant to any termination For Cause or pursuant to any termination for insufficient performance, as determined by the Company.
“RSA” means Stock subject to restrictions requiring that it be redelivered or offered for sale to the Company if specified service or performance-based conditions are not satisfied.
“RSU” means an unfunded and unsecured promise, denominated in shares of Stock, to deliver Stock or cash measured by the value of Stock in the future, subject to the satisfaction of specified performance or other vesting conditions.
“SAR” means the right to receive upon exercise an amount (payable in cash or in shares of Stock of equivalent value) equal to any excess in value of shares of Stock subject to the right over the base value from which appreciation is measured.
“Section 162(m)” means Section 162(m) of the Code, including the Treasury Regulations thereunder and other applicable Internal Revenue Service guidance.
“Section 409A” means Section 409A of the Code, including the Treasury Regulations thereunder and other applicable Internal Revenue Service guidance.
“Section 422” means Section 422 of the Code, including the Treasury Regulations thereunder and other applicable Internal Revenue Service guidance.
“Stock” means the common stock, $0.0005 par value, of the Company.
| | | | | B-13 | |  | |  |
 
BIOGEN INC. 225 BINNEY STREET CAMBRIDGE, MA 02142 VOTE BY INTERNET Before The Meeting- Go towww.proxyvote.com Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 P.M. Eastern Time the day before the meeting date. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. During The Meeting - Go towww.virtualshareholdermeeting.com/BIIB2017BIIB2019 You may attend the Meeting via the Internet and vote during the Meeting. Have the information that is printed in the box marked by the arrow available and follow the instructions. VOTE BY PHONE -1-800-690-6903 Use any touch-tone telephone to transmit your voting instructions up until 11:59 P.M. Eastern Time the day before the meeting date. Have your proxy card in hand when you call and then follow the instructions. VOTE BY MAIL Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717. | | | TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: | | E25472-P92180E76108-P22124 KEEP THIS PORTION FOR YOUR RECORDS | — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — |
DETACH AND RETURN THIS PORTION ONLY THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | BIOGEN INC. | | | | | | | | | | | | | | | | | | | | | | | | The Board recommends a voteFOR the following proposals: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 1. | | Election of Directors. To elect the elevenfourteen director nominees numbered 1a through 1k1n to serve for aone-year term extending until the 20182020 annual meeting of stockholders and their successors are duly elected and qualified. | | For | | Against | | Abstain | | | | | | | | | | | | For | | Against | | Abstain | | | | | | | | | | | | | | | | | | | | | | | 1a. 1b. 1c. 1d. 1e. 1f. 1g. 1h. 1i. | | John R. Chiminski Alexander J. Denner Caroline D. Dorsa William A. Hawkins Nancy L. Leaming Jesus B. Mantas Richard C. Mulligan Robert W. Pangia Stelios Papadopoulos Brian S. Posner
Eric K. Rowinsky
Lynn Schenk
| | ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ | | ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ | | ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ | | | | 1j. 1k. 1l. 1m. 1n. | | Brian S. Posner Eric K. Rowinsky Lynn Schenk Stephen A. Sherwin Michel Vounatsos | | | | ☐ | | ☐ | | ☐ | | | | | | | | | | | | | | | | 1k.☐
| | Michel Vounatsos☐
| | | | ☐ ☐ | | ☐ ☐ ☐ ☐ ☐ | | ☐ ☐ ☐ ☐ ☐ | | | | | | | | | | | | | | 2. | | To ratify the selection of PricewaterhouseCoopers LLP as Biogen Inc.’s independent registered public accounting firm for the fiscal year ending December 31, 2017.2019. | | ☐ | | ☐ | | ☐ | | | | | | | | | | | | | | | | 3. | | Say on Pay - To approve an advisory vote on executive compensation. | | ☐ | | ☐ | | ☐ | | | | | | | | | | | | | | The Board recommends a vote for1 YEAR on the following proposal:
| | 1 Year
| | 2 Years
| | 3 Years
| | Abstain
| | | | | | | | | | | | | | | 4.
| | Say When on Pay - To approve an advisory vote on the frequency of the advisory vote on executive compensation.
| | ☐
| | ☐
| | ☐
| | ☐
| | | | | | | | | | | | | | | | | | | The Board recommends a voteFOR the following proposal: | | For | | Against | | Abstain | | | | | | | | | | | | | | | | | | | 5.
| | To approve the Biogen Inc. 2017 Omnibus Equity Plan.
| | ☐
| | ☐
| | ☐
| | | | | | | | | | | | | | | | | | | 6.
| | To transact such other business as may be properly brought before the meeting and any adjournments or postponements thereof.
| | | | | | | | | | | | | | | | | | | | Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized officer. | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Signature [PLEASE SIGN WITHIN BOX] | | | | Date | | | | | | | | | | Signature (Joint Owners) | | Date | | | | | | |
V.1.2
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting: The 20172019 Notice and Proxy Statement and 20162018 Annual Report with Form10-K are available at:www.proxyvote.com. — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — E25473-P92180E76109-P22124
BIOGEN INC. Annual Meeting of Stockholders June 7, 2017,19, 2019, 9:00 AM,a.m. Eastern Daylight Time This proxy is solicited by the Board of Directors The undersigned hereby appoints Michel Vounatsos, Paul J. Clancy,Jeffrey D. Capello and Susan H. Alexander, and each of them (with full power to act alone), as proxies of the undersigned with all the powers the undersigned would possess if present during the 20172019 Annual Meeting,and with full power of substitution in each of them to appear, represent and vote all shares of common stock of Biogen Inc. which the undersigned would be entitled to vote at the 20172019 Annual Meeting of Stockholders, to be held at Biogen Inc.’s offices located at 225 Binney Street, Cambridge, Massachusetts 02142 and online at www.virtualshareholdermeeting.com/BIIB2017BIIB2019 on Wednesday, June 7, 2017,19, 2019, at 9:00 a.m. Eastern Daylight Time, and at any adjournment or postponement thereof. The shares represented by this proxy will be voted as directed herein. If no direction is indicated, such shares will be voted FOR the election of all of the director nominees listed in Proposal 1 and FOR Proposals 2 3 and 5, and for the 1 YEAR option for Proposal 4.3. As to any other matter that may be properly brought before the meeting or any adjournment or postponement thereof, proxy holders will vote in accordance with their best judgment. Continued and to be signed on reverse side V.1.2
|
|
|
|












 By Internet. You may vote atwww.proxyvote.com, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or,
By Internet. You may vote atwww.proxyvote.com, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, 
 By Telephone. You may vote using a touch-tone telephone by calling1-800-690-6903, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or,
By Telephone. You may vote using a touch-tone telephone by calling1-800-690-6903, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, 
 By Mail.If you received printed proxy materials, you may submit your vote by completing, signing and dating each proxy card received and returning it in the prepaid envelope. Sign your name exactly as it appears on the proxy card. Proxy cards submitted by mail must be received no later than June
By Mail.If you received printed proxy materials, you may submit your vote by completing, signing and dating each proxy card received and returning it in the prepaid envelope. Sign your name exactly as it appears on the proxy card. Proxy cards submitted by mail must be received no later than June 
 During the Annual Meeting. You may vote during the Annual Meeting by submitting a written ballot in person at the Annual Meeting. To obtain directions to attend the Annual Meeting, please contact our Investor Relations department at (781)464-2442. We will pass out ballots at the Annual Meeting to anyone who wishes to vote in person.
During the Annual Meeting. You may vote during the Annual Meeting by submitting a written ballot in person at the Annual Meeting. To obtain directions to attend the Annual Meeting, please contact our Investor Relations department at (781)464-2442. We will pass out ballots at the Annual Meeting to anyone who wishes to vote in person.


































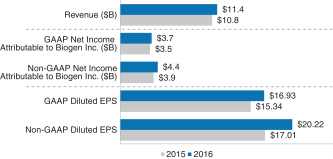


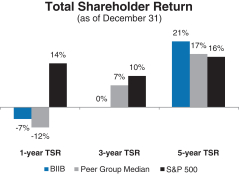
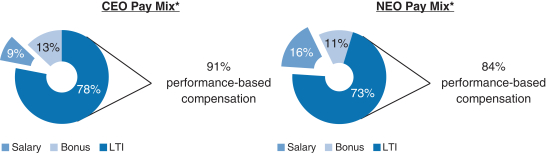




 Target Setting
Target Setting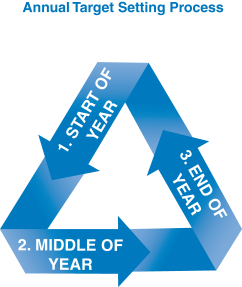


 Monitoring & Tracking
Monitoring & Tracking
 Results & Awards:
Results & Awards: